Abstract
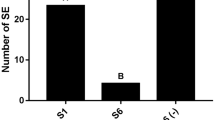
Differences in competence acquisition and subsequent embryo maturation in embryogenic and non-embryogenic callus of sugarcane var. SP79-1011 were evaluated using histomorphological analysis, growth curves, numbers of somatic embryos, and polyamine contents. Embryogenic callus was formed by cells with embryogenic characteristics such as a rounded shape, prominent nuclei, a high nucleus: cytoplasm ratio, small vacuoles and organized globular structures. However, non-embryogenic callus presented dispersed, elongated and vacuolated cells with a low nucleus: cytoplasm ratio; these characteristics did not allow for the development of somatic embryos even upon exposure to a maturation stimulus. These results suggest that non-embryogenic callus does not acquire embryogenic competence during induction and that maturation treatment is not sufficient to promote somatic embryo differentiation. The use of activated charcoal (AC; 1.5 g L−1) resulted in a higher somatic embryo maturation rate in embryogenic callus but did not yield success in non-embryogenic callus. Embryogenic callus incubated with control (10 μM 2,4-dichlorophenoxyacetic acid) and maturation (1.5 g L−1 AC) treatments for 28 days showed similar patterns of total free polyamines; these results differed from the results observed with non-embryogenic callus, suggesting that embryogenic callus already exhibits a characteristic pattern of endogenous polyamine levels. At 28 days of culture with maturation treatment, embryogenic callus exhibited significantly higher levels of free Spm than embryogenic callus incubated with control treatment and non-embryogenic callus incubated with both treatments. This result suggests that Spm could be important for the acquisition of embryogenic competence and somatic embryo maturation in sugarcane var. SP79-1011.








Similar content being viewed by others
References
Alcázar R, Altabella T, Marco F, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Arnaldos TL, Muñoza R, Ferrer MA, Calderón AA (2001) Changes in phenol content during strawberry (Fragaria × ananassa, cv. Chandler) callus culture. Physiol Plant 113:315–322
Arruda P (2011) Perspective of the sugarcane industry in Brazil. Trop Plant Biol 4:3–8
Arruda P (2012) Genetically modified sugarcane for bioenergy generation. Curr Opin Biotechnol 23:315–322
Baron K, Stasolla C (2008) The role of polyamines during in vivo and in vitro development. In Vitro Cell Dev Biol Plant 44:384–395
Bertoldi D, Tassoni A, Martinelli L, Bagni N (2004) Polyamines and somatic embryogenesis in two vitis vinifera cultivars. Physiol Plant 120:657–666
Blanco MA, Nieves N, Sanchez M, Borroto CG, Castillo R, González JL, Escalona M, Báez E, Hernández Z (1997) Protein changes associated with plant regeneration in embryogenic calli of sugarcane (Saccharum sp.). Plant Cell, Tissue Organ Cult 51:153–158
Burrieza HP, Lopez-Fernandez MP, Chiquieri TB, Silveira V, Maldonado SB (2012) Accumulation pattern of dehydrins during sugarcane (var. SP80.3280) somatic embryogenesis. Plant Cell Rep 31:2139–2149
Butterfield MK, Thomas DW (1996) Sucrose, yield and disease resistance characteristics of sugarcane varieties under test in the SASEX selection programme. Proc S Afr Sug Technol Ass 70:103–105
Butterfield MK, D’Hont A, Berding N (2001) The sugarcane genome: a synthesis of current understanding, and lessons for breeding and biotechnology. Proc S Afr Sug Technol Ass 75:1–5
Cangahuala-Inocente GC, Steiner N, Santos M, Guerra MP (2004) Morphohistological analysis and histochemistry of Feijoa sellowiana somatic embryogenesis. Protoplasma 224:33–40
Ceccarelli N, Mondin A, Curadi M, Lorenzi R, Schiavo FL (2000) Auxin metabolism and transport in an embryogenic cell line of Daucus carota L. J Plant Physiol 157:17–23
Chugh A, Khurana P (2002) Gene expression during somatic embryogenesis-recent advances. Curr Sci 86:715–730
Cidade DAP, Garcia RO, Duarte AC, Sachetto-Martins G, Mansur E (2006) Morfogênese in vitro de variedades brasileiras de cana-de-açúcar. Pesq Agropec Bras 41:385–391
Dalal MA, Sharma BB, Rao S (1992) Studies on stock plant treatment and initiation culture model in control of oxidative browning in in vitro cultures of grapevine. Sci Hortic 51:35–41
Desai NS, Suprasanna P, Bapat VA (2004) Simple and reproducible protocol for direct somatic embryogenesis from cultured immature inflorescence segments of sugarcane (Saccharum spp.). Curr Sci 87:764–768
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
El Meskaoui A, Trembaly FM (2009) Effects of exogenous polyamines and inhibitors of polyamine biosynthesis on endogenous free polyamine contents and the maturation of white spruce somatic embryos. Afr J Biotech 8:6807–6816
Falco MC, Mendes BMJ, Neto AT, Glória BA (1996) Histological characterization of in vitro regeneration of Saccharum sp. Rev Bras Fisiol Veg 8:93–97
FAO (Food and Agriculture Organization of the United Nations) (2012) FAO statistical database. http://www.faostat.org.br. Accessed 3 October 2012
Fehér A, Pasternak T, Miskolczi P, Ayaydin F, Dudits D (2001) Induction of the embryogenic pathway in somatic plant cells. Acta Hortic 560:293–298
Fehér A, Pasternak T, Otvos K, Miskolczi P, Dudits D (2002) Induction of embryogenic competence in somatic plant cells: a review. Biologia 57:5–12
Fehér A, Pasternak T, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell, Tissue Organ Cult 74:201–228
Gahan PB, George EF (2008) Adventitious regeneration. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture, 3rd edn. Springer, Dordrecht, pp 355–402
Gallo-Meagher M, English RG, Abouzid A (2000) Thidiazuron stimulates shoot regeneration of sugarcane embryogenic callus. In Vitro Cell Dev Biol Plant 36:37–40
Gandonou CH, Errabii T, Abrini J, Idaomar M, Chibi F, Senhaji NS (2005) Effect of genotype on callus induction and plant regeneration from leaf explants of sugarcane (Saccharum sp.). Afr J Biotechnol 4:1250–1255
Hadrami I, D’auzac J (1992) Effects of growth regulators on polyamine content and peroxidase activity in Hevea brasiliensis callus. Ann Bot 69:323–325
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt EDL, Boutilier K, Grossniklaus U, de Vries SC (2001) The arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816
Ho WJ, Vasil IK (1983) Somatic embryogenesis in sugarcane (Saccharum officinarum L.): growth and plant regeneration from embryogenic cell suspension cultures. Ann Bot 51:7–726
Jimenez VM (2001) Regulation of in vitro somatic embryogenesis with emphasis on the role of endogenous hormones. Rev Bras Fisiol Veg 13:196–223
Jimenez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–110
Karami O, Saidi A (2010) The molecular basis for stress-induced acquisition of somatic embryogenesis. Mol Biol Rep 37:2493–2507
Karami O, Aghavaisi B, Pour AM (2009) Molecular aspects of somatic-to-embryogenic transition in plants. J Chem Biol 2:177–190
Kuznetsov V, Shorina M, Aronova E, Stetsenko L, Rakitin V, Shevyakova NI (2007) NaCl- and ethylene-dependent cadaverine accumulation and its possible protective role in the adaptation of the common ice plant to salt stress. Plant Sci 172:363–370
Lakshmanan P (2006) Somatic embryogenesis in sugarcane. In Vitro Cell Dev Biol Plant 42:201–205
Lakshmanan P, Geijskes RJ, Aitken KS, Grof CLP, Bonnett GD, Smith GR (2005) Sugarcane biotechnology: the challenges and opportunities. In Vitro Cell Dev Biol Plant 41:345–363
Lameira AO, Pinto JEBP, Arrigoni-Blank MF, Cardoso MG (1997) Efeito de compostos fenólicos, carvão ativado e do meio físico no desenvolvimento de segmento nodal de Cordia verbenacea L. Cienc Rural 27:189–192
Li Z, Burrit DJ (2003) Changes in endogenous polyamines during the formation of somatic embryos from isogenic lines of Dactylis glomerata L. with different regenerative capacities. Plant Growth Regul 40:65–74
Lima MAC, Garcia RO, Sachetto-Martins G, Mansur E (2001) Morfogênese in vitro e susceptibilidade de calos de variedades nacionais de cana-de-açúcar (Saccharum officinarum L.) a agentes seletivos utilizados em sistemas de transformação genética. Rev Bras Bot 24:73–77
LoSchiavo F, Filippini F, Cozzani F, Vallone D, Terzi M (1991) Modulation of auxin-binding proteins in cell suspensions I differential responses of carrot embryo cultures. Plant Physiol 97:60–64
McCabe PF, Valentine TA, Fosberg LS, Pennel RI (1997) Soluble signals from cells identified at the cell wall establish a developmental pathway in carrot. Plant Cell 9:2225–2241
Michalczuk L, Ribnicky DM, Cooke TJ, Cohen JD (1992) Regulation of indole-3-acetic acid biosynthetic pathways in carrot cell cultures. Plant Physiol 100:1346–1353
Minocha R, Dale RS, Cathie R, Steelec KD, Minocha SC (1999) Polyamine levels during the development of zygotic and somatic embryos of Pinus radiata. Physiol Plant 105:155–164
Minocha R, Minocha SC, Long S (2004) Polyamines and their biosynthetic enzymes during somatic embryo development in red spruce (Picea rubens Sarg.). In Vitro Cell Dev Biol Plant 40:572–580
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nieves N, Segura-Nieto M, Blanco MA, Sanchez M, Gonzalez A, Gonzalez JL, Castillo R (2003) Biochemical characterization of embryogenic and non-embryogenic calluses of sugarcane. In Vitro Cell Dev Biol Plant 39:343–345
Nieves N, Sagarra F, González R, Lezcano Y, Cid M, Blanco MA, Castillo R (2008) Effect of exogenous arginine on sugarcane (Saccharum sp.) somatic embryogenesis, free polyamines and the contents of the soluble proteins and proline. Plant Cell, Tissue Organ Cult 95:313–320
Pan Z, Zhu S, Guan R, Deng X (2010) Identification of 2,4-d-responsive proteins in embryogenic callus of Valencia sweet orange (Citrus sinensis Osbeck) following osmotic stress. Plant Cell, Tissue Organ Cult 103:145–153
Patade VY, Suprasanna P, Bapat VA (2008) Effects of salt stress in relation to osmotic adjustment on sugarcane (Saccharum officinarum L.) callus cultures. Plant Growth Regul 55:169–173
Paul A, Mitter K, Raychaudhuri SS (2009) Effect of polyamines on in vitro somatic embryogenesis in Momordica charantia L. Plant Cell, Tissue Organ Cult 97:303–311
Pennell RI, Graham NLJ, Hilbert S, de Vries SC, Robertsw K (1992) Identification of a transitional cell state in the developmental pathway to carrot somatic embryogenesis. J Cell Biol 119:1371–1380
Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell, Tissue Organ Cult 86:285–301
Rodriguez S, Mondejar C, Ramos ME, Diaz E, Maribona R, Ancheta O (1995) Sugarcane somatic embryogenesis: a scanning electron microscopy study. Tissue Cell 28:149–154
Santa-Catarina C, Hanai LR, Dornelas MC, Viana AM, Floh EIS (2004) SERK gene homolog expression, polyamines and amino acids associated with somatic embryogenic competence of Ocotea catharinensis Mez. (Lauraceae). Plant Cell, Tissue Organ Cult 79:53–61
Santa-Catarina C, Silveira V, Balbuena TS, Viana AM, Estelita MEM, Handro W, Floh EIS (2006) IAA, ABA, polyamines and free amino acids associated with zygotic embryo development of Ocotea catharinensis. Plant Growth Regul 49:237–247
Santa-Catarina C, Silveira V, Scherer GFE, Floh EIS (2007) Polyamines and nitric oxide induce morphogenetic evolution in somatic embryogenesis of Ocotea catharinensis. Plant Cell, Tissue Organ Cult 90:93–101
Sasaki K, Shimomura K, Kamada H, Harada H (1994) IAA metabolism in embryogenic and non-embryogenic carrot cells. Plant Cell Physiol 35:1159–1164
Shoeb F, Yadav JS, Bajaj S, Rajam MV (2001) Polyamines as biomarkers for plant regeneration capacity: improvement of regeneration by modulation of polyamine metabolism in different genotypes of Indica rice. Plant Sci 160:1229–1235
Silveira V, Balbuena TS, Santa-Catarina C, Floh EIS, Guerra MP, Handro W (2004) Biochemical changes during seed development in Pinus taeda L. Plant Growth Regul 44:147–156
Silveira V, Santa-Catarina C, Tun NN, Scherer GFE, Handro W, Guerra MP, Floh EIS (2006) Polyamine effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryogenic suspension cultures of Araucaria angustifolia (Bert.) O Ktze. Plant Sci 171:91–98
Snyman SJ, Meyer GM, Koch AC, Banasiak M, Watt MP (2011) Applications of in vitro culture systems for commercial sugarcane production and improvement. In Vitro Cell Dev Biol Plant 47:234–249
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman and Co, New York
Steiner N, Santa-Catarina C, Silveira V, Floh EIS, Guerra MP (2007) Polyamine effects on growth and endogenous hormones levels in Araucaria angustifolia embryogenic cultures. Plant Cell, Tissue Organ Cult 89:55–62
Steiner N, Santa-Catarina C, Andrade JBR, Balbuena TS, Guerra MP, Handro W, Floh EIS, Silveira V (2008) Araucaria angustifolia biotechnology—review. Func Plant Sci Biotechnol 2:20–28
Steiner N, Santa-Catarina C, Guerra MP, Cutri L, Dornelas MC, Floh EIS (2012) A gymnosperm homolog of SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE-1 (SERK1) is expressed during somatic embryogenesis. Plant Cell, Tissue Organ Cult 109:41–50
Taparia Y, Gallo M, Altpeter F (2012) Comparison of direct and indirect embryogenesis protocols, biolistic gene transfer and selection parameters for efficient genetic transformation of sugarcane. Plant Cell, Tissue Organ Cult 111:131–141
Tautorus TE, Fowke LC, Dunstan DI (1991) Somatic embryogenesis in conifers. Can J Bot 69:1873–1899
Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26:618–631
Toonen MAJ, Schmidt EDL, van Kammen A, de Vries SC (1997) Promotive and inhibitory effects of diverse arabinogalactan proteins on Daucus carota L. somatic embryogenesis. Planta 203:188–195
Verdeil JL, Alemanno L, Niemenak N, Trangarder TJ (2007) Pluripotent versus totipotent plant stem cells: dependence versus autonomy. Trends Plant Sci 12:245–252
Wallace HM, Fraser AV, Hughes A (2003) A perspective of polyamine metabolism. Biochem J 376:1–14
Wu XB, Wang J, Liu JH, Deng XX (2009) Involvement of polyamine biosynthesis in somatic embryogenesis of Valencia sweet orange (Citrus sinensis) induced by glycerol. J Plant Physiol 166:52–62
Zavattieri MA, Frederico AM, Lima M, Sabino R, Arnholdt-Schmitt B (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron J Biotechnol 13:4
Zeng F, Zhang X, Cheng L, Hu L, Zhu L, Cao J, Guo X (2007) A draft gene regulatory network for cellular totipotency reprogramming during plant somatic embryogenesis. Genomics 90:620–628
Zhang BH, Liu F, Yao CB (2000) Plant regeneration via somatic embryogenesis in cotton. Plant Cell, Tissue Organ Cult 60:89–94
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Acknowledgments
Funding for this work was provided by the CNPq (403015/2008-1 and 480142/2010-6) and FAPERJ (E-26/101.513/2010) to VS. AMV thanks CAPES for her fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

11240_2013_330_MOESM1_ESM.jpg
Somatic embryo regeneration and plantlet germination during somatic embryogenesis of sugarcane var. SP 79-1011. A somatic embryo obtained from the maturation treatment containing 1.5 g L−1 AC (A), a plantlet regenerated from a somatic embryo in a culture flask containing 20 mL of MS culture medium supplemented with sucrose (20 g L−1) and Phytagel® (2 g L−1) (B). The arrow in (C) indicates plantlet root formation. Bars: A = 0.4 cm, B and C = 2.0 cm. (JPEG 1461 kb)

11240_2013_330_MOESM2_ESM.jpg
Histomorphology of sugarcane callus containing portions of embryogenic and non-embryogenic callus. The images show morphology as viewed by light stereoscopy (A), morphology as viewed by scanning electron microscopy (B) and histology as viewed by light microscopy (C). E = embryogenic. NE = non-embryogenic. Bars: A = 3.5 mm; B = 1 mm.; C = 200 μm. (JPEG 3824 kb)
Rights and permissions
About this article
Cite this article
Silveira, V., de Vita, A.M., Macedo, A.F. et al. Morphological and polyamine content changes in embryogenic and non-embryogenic callus of sugarcane. Plant Cell Tiss Organ Cult 114, 351–364 (2013). https://doi.org/10.1007/s11240-013-0330-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0330-2