Abstract
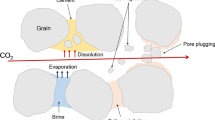
Although there are a number of mathematical modeling studies for carbon dioxide (CO2) injection into aquifer formations, experimental studies are limited and most studies focus on injection into sandstone reservoirs as opposed to carbonate ones. This study presents the results of computerized tomography (CT) monitored laboratory experiments to analyze permeability and porosity changes as well as to characterize relevant chemical reactions associated with injection and storage of CO2 in carbonate formations. CT monitored experiments are designed to model fast near well bore flow and slow reservoir flows. Highly heterogeneous cores drilled from a carbonate aquifer formation located in South East Turkey were used during the experiments. Porosity changes along the core plugs and the corresponding permeability changes are reported for different CO2 injection rates and different salt concentrations of formation water. It was observed that either a permeability increase or a permeability reduction can be obtained. The trend of change in rock properties is very case dependent because it is related to distribution of pores, brine composition and thermodynamic conditions. As the salt concentration decreases, porosity and the permeability decreases are less pronounced. Calcite deposition is mainly influenced by orientation, with horizontal flow resulting in larger calcite deposition compared to vertical flow.
Similar content being viewed by others
References
Akin, S., Kovscek, A.R.: Computed tomography in petroleum research. In: Mees, F., Swennen, R., Van Geet, M., Jacobs P. (eds.) Application of X-ray Computed Tomography in the Geosciences, pp. 23–38. Geological Society of London (2003)
Bachu S. and Adams J.J. (2003). Sequestration of CO2 in geological media in response to climate change: capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manage. 44: 3151–3175
Bartels, J., Kühn, M., Schneider, W., Clauser, C., Pape, H., Meyn, V., Lajczak, I.: Core flooding laboratory experiment validates numerical simulation of induced permeability change in reservoir sandstone. Geophys. Res. Lett. 29(9), 10.1029/2002GL014901 (2002)
Bekri S., Thovert J.F. and Adler P.M. (1995). Dissolution of porous media. Chem. Eng. Sci. 50: 2765–2791
Bernabe Y., Mok U. and Evans B. (2003). Permeability–porosity relationships in rocks subjected to various evolution processes. Pure Appl. Geophys. 160: 937–960
Bhat S.K. and Kovscek A.R. (1999). Statistical network theory of silica deposition and dissolution in diatomite. In Situ 23: 21–53
Bjorlykke K. (1989). Sedimentology and Petroleum Geology. Springer-Verlag, New York
Clauser C. (ed) (2003). Numerical Simulation of Reactive Flow in Hot Aquifers using SHEMAT/Processing Shemat. Springer Verlag, Heidelberg-Berlin
Computer Modeling Group (CMG): CMG STARS User’s Guide. Computer Modeling Group Ltd., Calgary, Alberta, Canada (2003)
David C., Wong T.-F., Zhu W. and Zhang J. (1994). Laboratory measurement of compaction-induced permeability change in porous rock: implications for the generation and maintenance of pore pressure excess in the crust. Pure Appl. Geophys. 143: 425–456
Diabira I., Castanier L.M. and Kovscek A.R. (2001). Porosity and permeability evolution accompanying hot fluid injection into diatomite. Petrol. Sci. Technol. 19(9&10): 1167–1185
Doughty, C., Pruess, K.: Modeling supercritical carbon dioxide injection in heterogeneous porous media. Vadose Zone Journal. 3(3), 837–847 (2004)
Gabriel, G.A., Inamdar, G.R.: An experimental investigation of fines migration in porous media. SPE 12168, 58th Annual Technical Conference and Exhibition, San Francisco, CA, October 5–8 (1983)
Goldberg, P., Chen, Z.-Y., O’Connor, W., Walters, R., Ziock, H.: CO2 mineral sequestration studies in US. In: Proceedings of the First National Conference on Carbon Sequestration, Washington, DC, U.S.A., May 14–17 (2001)
Gunter W.D., Perkins E.H. and Hutcheon I. (2000). Aquifer disposal of acid gases: modelling of water–rock reactions for trapping of acid wastes. Appl. Geochem. 15: 1085–1095
Gunter, W.D., Perkins, E.H., McCann, T.J.: Aquifer disposal of CO2-rich gases: reaction design for added capacity. Energ. Convers. Manage. 37, 1135–1142 (1993)
Gunter W.D., Wiwchar B. and Perkins E.H. (1997). Aquifer disposal of CO2-rich greenhouse gases: extension of the time scale of experiment for CO2-sequestering reactions by geochemical modelling. Mineral. Petrol. 59: 121–140
Hibbeler, J., Garcia, T., Chavez, N.: An integrated long term solution for migratory fines damage. SPE Latin American and Caribbean Petroleum Engineering Conference, Port of Spain, Trinidad, West Indies, 27–30 April (2003)
Holtz, H.M.: Pore-scale influences on saline aquifer CO2 sequestration. AAPG 2003 Meeting, Salt Lake City, Utah, May 11–14 (2003)
Hovorka, S.D., Benson, S.M., Doughty, C., Freifeld, B.M., Sakurai, S., Daley, T.M., Kharaka, Y.K., Holtz, M.H., Trautz, R.C., Nance, H.S., Myer, L.R., Knauss, K.G.: Measuring permanence of CO2 storage in saline formations -the Frio experiment. Environ. Geosci. 13, 105–121 (2006)
Itoi, R., Fukuda, M., Jinno, K., Shimizu, S., Tomita, T.: Numerical analysis of the injectivity of wells in the Otake geothermal field, Japan. In: Proceedings 9th New Zealand Geothermal Workshop, November 4–6, Geothermal Institute, University of Auckland, Auckland, New Zealand, pp. 103–108 (1987)
Izgec, O., Demiral, B., Bertin, H., Akin, S.: Calcite precipitation in low temperature geothermal systems: an experimental approach. In: 30th Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, California, TR-176 (2005a)
Izgec, O., Demiral, B., Bertin, H., Akin, S.: CO2 injection in carbonates. In: SPE Western Regional Meeting, Irvine, CA, USA, SPE Paper 93773 (2005b)
Izgec, O., Demiral, B., Bertin, H., Akin, S.: Experimental and numerical investigation of carbon sequestration in deep saline aquifers. In: SPE/EPA/DOE Exploration and Production Environmental Conference, Galveston, Texas, SPE Paper 94697 (2005c)
Izgec, O., Demiral, B., Bertin, H., Akin, S.: CO2 injection into saline carbonate aquifer formations II: comparison of numerical simulations to experiments Transport Porous Media (to appear) (2007)
Johnson J.W., Nitao J.J., Morris J.P.: Reactive transport modeling of cap rock integrity during natural and engineered CO2 sequestration. Abstr. Pap. Am. Chem. S., 226: U604-U604 137-GEOC Part 1. (2003)
Kaszuba J.P. and Janecky D.R. (2000). Experimental hydration and carbonation reactions of MgO: a simple analog for subsurface carbon sequestration processes. Geol. Soc. Am., Abstr. with Prog. 32: A202
Kaszuba J.P., Janecky D.R. and Snow M.G. (2001). Carbon dioxide reaction processes in a model brine aquifer at 200 C and 200 bars: implications for subsurface carbon sequestration. Geol. Soc. Am., Abstr. with Prog. 33: A232
Kaszuba J.P., Janecky D.R. and Snow M.G. (2003). Carbon dioxide reaction processes in a model brine aquifer at 200^C and 200 bars: implications for geologic sequestration of carbon. Appl. Geochem. 18: 1065–1080
Kumar, A., Ozah, R., Noh, M., Pope, G.A., Bryant, S., Sepehrnoori, K., Lake, L.W.: Reservoir simulation of CO2 storage in deep saline aquifers. SPE J. 10 (3): 336–348 (2005)
Kühn M. (2004). Reactive flow modeling of hydrothermal systems. Lect. Notes Earth Sci. 103: 209–226
Lichtner P.C. (1996). Continuum formulation of multicomponent – multiphase reactive transport. Rev. Mineral. Geochem. 34(1): 83–129
McCume C.C., Forgler H.S. and Kline W.E. (1979). An experiment technique for obtaining permeability–porosity relationship in acidized porous media. Ind. Eng. Chem. Fundam. 18(2): 188–192
McPherson, B.J.O.L., Lichtner, P.C.: CO2 sequestration in deep aquifers. In: Proceedings of the First National Conference on Carbon Sequestration, Washington, DC, U.S.A., May 14–17 (2001)
Moghadasi J., Müller-Steinhagen H., Jamialahmadi M. and Sharif A. (2005). Model Study on the kinetics of oil formation damage due to salt precipitation from injection. J. Petrol. Sci. Eng. 46(4): 299–299
Nghiem, L., Sammon, P., Grabenstetter, J., Ohkuma, H.: Modeling CO2 storage in aquifers with a fully-coupled geochemical EOS compositional simulator. SPE 89474-MS, SPE/DOE Symposium on Improved Oil Recovery, Tulsa, Oklahoma., April 17–21 (2004)
Nordbotten M.J., Celia A.M. and Bachu S. (2004). Injection and storage of CO2 in deep saline aquifers: analytical solution for CO2 plume evolution during injection. Transport Porous Media 58(3): 339–360
Omole, O., Osoba, J.S.: Carbondioxide–dolomite rock interaction during CO2 flooding process. In: 34th Annual Technical Meeting of the Petroleum Society of CIM, Canada (1983)
Pange M.K.R. and Ziauddin M. (2005). Two-scale continuum model for simulation of wormholes in carbonate acidization. AIChE J. 51(12): 3231–3248
Parkhurst, D.L., Appelo, C.A.J.: User’s guide to PHREEQC (Version 2) – a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Water-Resources Investigations Report 99-4259, 312 pp (1999)
Perkins, E.H., Gunter, W.D.: Aquifer disposal of CO2-rich greenhouse gasses: modelling of water–rock reaction paths in a siliciclastic aquifer. In: Kharaka, Y.K., Chudaev, O.V. (eds.) Proceedings of the 8th International Symposium on Water–Rock Interaction, pp. 895–898 (1995)
Pruess, K., Xu, T.: Numerical modeling of aquifer disposal of CO2. In: SPE/EPA/DOE Exploration and Production Environmental Conference, San Antonio, Texas, SPE Paper 83695 (2001)
Pruess, K., Xu T.F., Apps, J., Garcia, J.: Numerical modeling of aquifer disposal of CO2. SPE J. 8(1), 49–60 (2003)
Quintard M. and Whitaker S. (1999). Dissolution of an immobile phase during flow in porous media. Ind. Eng. Chem. Res. 38: 833–844
Reichle, D., Houghton, J., Benson, S., Clarke, J., Dahlman, R., Hendrey, G., Herzog, H., Hunter-Cevera, J., Jacobs, G., Judkins, R., Kane, B., Ekmann, J., Ogden, J., Palmisano, A., Socolow, R., Stringer, J., Surles, T., Wolsky, A., Woodward, N., York, M.: Carbon Sequestration Research and Development, Office of Science, Office of Fossil Energy, U.S. Department of Energy (1999)
Ross, G.D., Todd, A.C., Tweedie, J.A., Will, A.G.S.: The dissolution effects of CO2–brine systems on the permeability of U.K. and North Sea Calcareous Sandstones. In: Proceedings of Society of Petroleum Engineers/U.S. Department of Energy Third Joint Symposium on Enhanced Oil Recovery, Paper SPE/DOE 10685, pp. 149–154 (1982)
Saripalli P. and McGrail P. (2002). Semi-analytical approaches to modeling deep well injection of CO2 for geological sequestration. Energy Convers. Manage. 43: 185–198
Snoeyink, L.W., Jenkins, D.: Water Chemistry, pp. 85–135. John Wiley & Sons Publications (1980)
Spycher N., Pruess K. and Ennis-King J. (2003). CO2–H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100°C and up to 600 bar. Geochim. Cosmochim. Acta 67(16): 3015–3031
Soong Y., Goodman A.L., McCarthy-Jones J.R. and Baltrus J.P. (2004). Experimental and simulation studies on mineral trapping of CO2 with brine. Energy Convers. Manage. 45: 1845–1859
Walsh J.B. and Brace W.F. (1984). The effect of pressure on porosity and the transport properties of rocks. J. Geophys. Res. 89: 9425–9431
Zarrouk, S.J., O’Sullivan, M.J.: The effect of chemical reactions on the transport properties of porous media. In: Simmons, S., Dunstall, M.G., Morgan, O.E. (eds.) Proceedings 23rd New Zealand Geothermal Workshop, November 7–9, Auckland University, New Zealand, pp. 231–236 (2001)
Zhang, D., Kang, Q.: Simulation of coupled flow, transport, and reaction in porous media by lattice Boltzmann method. In: 2004 AGU Fall Meeting, San Francisco, U.S.A. H32A-06 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Izgec, O., Demiral, B., Bertin, H. et al. CO2 injection into saline carbonate aquifer formations I: laboratory investigation. Transp Porous Med 72, 1–24 (2008). https://doi.org/10.1007/s11242-007-9132-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-007-9132-5