Abstract
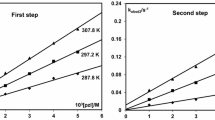
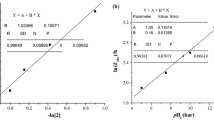
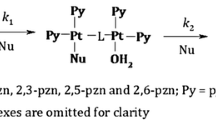
The reaction between Pd(N,N′)Cl2 [N,N′ ≡ 1-alkyl-2-(arylazo)imidazole (N,N′) and picolinic acid (picH) have been studied spectrophotometrically at λ = 463 nm in MeCN at 298 K. The product is [Pd(pic)2] which has been verified by the synthesis of the pure compound from Na2[PdCl4] and picH. The kinetics of the nucleophilic substitution reaction have been studied under pseudo-first-order conditions. The reaction proceeds in a two-step-consecutive manner (A → B → C); each step follows first order kinetics with respect to each complex and picH where the rate equations are: Rate 1 = {k′0 + k′2[picH]0} × [Pd(N,N′)Cl2] and Rate 2 = {k′′0 + k′′2[picH]0}[Pd(N,O)(monodentate N,N′)Cl2] such that the first step second order rate constant (k′2) is greater than the second step second order rate constant (k′′2). External addition of Cl− (as LiCl) suppresses the rate. Increase in π-acidity of the N,N′ ligand, increases the rate. The reaction has been studied at different temperatures and the activation parameters (Δ‡H° and Δ‡S°) were calculated from the Eyring plot.
Similar content being viewed by others
References
S.J. Lippard, in: I. Bertini, H.B. Gray, S.J. Lippard and J.S.Valentine (eds) Bioinorganic Chemistry, University Science Books, Mill Valley, CA, 1994, p. 505; P.D. Beer, Acc. Chem. Res., 31, 71 (1998); Y.S. Yoo, Y.S. Sohn and Y.K. Do, J. Inorg. Biochem. 73, 187 (1999).
J. Li L.M. Zheng I. King T.W. Doyle S.H. Chan (2001) Curr. Med. Chem. 8 121 Occurrence Handle1:CAS:528:DC%2BD3MXhtVSgtro%3D
P. Banerjee, Coord. Chem. Rev., 19, 190 (1999); B. Lippert, Coord. Chem. Rev., 18, 263 (1999); M. Kato, J. Takahashi, Y. Sugimoto, C. Kosuge, S. Kishi and S.J . Yano, J. Chem. Soc. Dalton Trans., 747 (2001).
H. Chen, J.A. Parkinson, S. Parsons, R.A. Coxall, R.O. Gould and P.J. Sadler, J. Am. Chem. Soc., 124, 3064 (2002); A.H. Velders, H. Kooijman, A.L. Spek, J.G. Hassnoot, D.D. Vos and J. Reedijk, Inorg. Chem., 39, 2966 (2000).
T.K. Misra, D. Das, C. Sinha, P.K. Ghosh and C.K. Pal, Inorg. Chem., 37, 1672 (1998); P. Byabartta, P.K. Santra, T.K. Misra, C. Sinha and C.H.L. Kennard, Polyhedron, 20, 905 (2001); P.K. Santra, T.K. Misra, D. Das, C. Sinha, A.M.Z. Slawin and J.D. Woollins, Polyhedron, 18, 2869 (1999).
S. Pal, D. Das, P. Chattopadhyay, C. Sinha, K. Panneerselvam and T.-H. Lu, Polyhedron, 19, 1263 (2000); G.K. Rauth, S. Pal, D. Das, C. Sinha, A.M.Z. Slawin and J.D. Woollins, Polyhedron, 20, 363 (2001).
R. Roy, P. Chattopadhyay, C. Sinha and S. Chattopadhyay, Polyhedron, 15, 3361 (1996); D. Das, M.K. Nayak and C. Sinha, Transition Met. Chem., 22, 172 (1997); D. Das and C. Sinha, Transition Met. Chem., 23, 309 (1998).
R. Roy, T.K. Misra, C. Sinha, A. Mahapatra and A. Sanyal, Transition Met. Chem., 22, 453 (1997); R. Roy, D. Das, M. Banerjee, C. Sinha and A. Mahapatra, Transition Met. Chem., 26, 205 (2001).
R. Roy D. Das A. Mahapatra C. Sinha (2000) Inorg. React. Mech. 2 213 Occurrence Handle1:CAS:528:DC%2BD3cXotVWhtL0%3D
G.K. Routh S.K. Jasimuddin A. Mahapatra C. Sinha (2002) Inorg. React. Mech. 5 31
S. Pal, T.K. Misra, P. Chattopadhyay and C. Sinha, Proc. Indian Acad. Sci. (Chem. Sci.), 111, 687 (1999); P. Byabartta, S. Pal, T.K. Misra, C. Sinha, F.-L. Liao, K. Panneerselvam and T.-H. Lu, J. Coord. Chem., 55, 479 (2002); S. Senapati, U.S. Ray, P.K. Santra, C. Sinha, J.D. Woollins and A.M.Z. Slawin, Polyhedron, 21, 753 (2002).
P. Atkins J.D. Paula (2002) Physical Chemistry EditionNumber7 Oxford University Press Oxford 956
S. Dey, M. Ray and P. Banerjee, Inorg. React. Mech., 2, 267 (2000); P.S. Sengupta, R. Sinha and G.S. De, Indian J. Chem., 40A, 509 (2001).
F. Fasalo and R.G. Pearson, Mechanism of Inorganic Reactions. A study of Metal Complexes in Solution, 2nd edit., Wiley, New York, 1967; R.G. Wilkins, Kinetics and Mechanism of Reactions of Transition Metal Complexes, 2nd edit., VCH, Weinheim, 1991
C. Sinha, Ph.D. thesis, Jadavpur University, Kolkata-32, India, 1990.
G.K. Rauth, S. Pal, D. Das, C. Sinha, A.M.Z. Slawin and J.D. Woollins, Polyhedron, 20, 363 (2001); S. Pal, D. Das, P. Chattopadhyay, C. Sinha, K. Panneerselvam and T.-H. Lu, Polyhedron, 19, 1263 (2000); J. Arpalahli and P. Lehikoinen, Inorg. Chem., 29, 2564 (1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saha, S., Sarkar, P.K. & Mahapatra, A. Kinetics and Mechanism of the Reactions of Picolinic Acid with Dichloro-{1-alkyl-2-(arylazo)imidazole}palladium(II) Complexes. Transition Met Chem 31, 389–395 (2006). https://doi.org/10.1007/s11243-006-0003-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11243-006-0003-7