Abstract
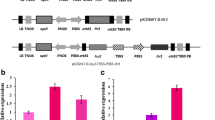
Cystatins from plants have been implicated in plant defense towards insects, based on their role as inhibitors of heterologous cysteine-proteinases. We have previously characterized thirteen genes encoding cystatins (HvCPI-1 to HvCPI-13) from barley (Hordeum vulgare), but only HvCPI-1 C68 → G, a variant generated by direct-mutagenesis, has been tested against insects. The aim of this study was to analyze the effects of the whole gene family members of barley cystatins against two aphids, Myzus persicae and Acyrthosiphon pisum. All the cystatins, except HvCPI-7, HvCPI-10 and HvCPI-12, inhibited in vitro the activity of cathepsin L- and/or B-like proteinases, with HvCPI-6 being the most effective inhibitor for both aphid species. When administered in artificial diets, HvCPI-6 was toxic to A. pisum nymphs (LC50 = 150 μg/ml), whereas no significant mortality was observed on M. persicae nymphs up to 1000 μg/ml. The effects of HvCPI-6 ingestion on A. pisum were correlated with a decrease of cathepsin B- and L-like proteinase activities. In the case of M. persicae, there was an increase of these proteolytic activities, but also of the aminopeptidase-like activity, suggesting that this species is regulating both target and insensitive enzymes to overcome the effects of the cystatin. To further analyze the potential of barley cystatins as insecticidal proteins against aphids, Arabidopsis plants expressing HvCPI-6 were tested against M. persicae. For A. pisum, which does not feed on Arabidopsis, a combined diet-Vicia faba plant bioassay was performed. A significant delay in the development time to reach the adult stage was observed in both species. The present study demonstrates the potential of barley cystatins to interfere with the performance of two aphid species.



Similar content being viewed by others
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Abraham Z, Martinez M, Carbonero P, Diaz I (2006) Structural and functional diversity within the cystatin gene family of Hordeum vulgare. J Exp Bot 57:4245–4255
Álvarez-Alfageme F, Martinez M, Pascual-Ruiz S, Castañera P, Diaz I, Ortego F (2007) Effects of potato plants expressing a barley cystatin on the predatory bug Podisus maculiventris via herbivorous prey feeding on the plant. Transgenic Res 16:1–13
Azzouz A, Cherqui A, Campan EDM, Rahbé Y, Duport G, Jouanin L, Kiser L, Giordanengo P (2005) Effects of plant protease inhibitors, oryzacystatin I and soybean Bowman-Birk inhibitor, on the aphid Macrosiphum euphorbiae (Homoptera, Aphididae) and its parasitoid Aphelinus abdominalis (Hymenoptera, Aphididae). J Insect Physiol 51:75–86
Baulcome DC, Saunders GR, Bevan MW, Mayo MA, Harrison BD (1986) Expression of biotechnologically active viral satellite RNA from the nuclear genome of transformed plants. Nature 321:446–449
Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T (2009) Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc 4:1–13
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cowgill SE, Wright C, Atkinson HJ (2002) Transgenic potatoes with enhanced levels of nematode resistance do not have altered susceptibility to nontarget aphids. Mol Ecol 11:821–827
Cristofoletti PT, Ribeiro AF, Deraison C, Rahbé Y, Terra WR (2003) Midgut adaptation and digestive enzyme distribution in a phloem feeding insect, the pea aphid Acythosiphon pisum. J Insect Physiol 49:11–24
Cristofoletti PT, Mendonça de Sousa FA, Rahbé Y, Terra WR (2006) Characterization of a membrane-bound aminopeptidase purified from Acyrthosiphon pisum midgut cells. A major binding site for toxic mannose lectins. FEBS J 273:5574–5588
Dutta I, Saha P, Majumder P, Sarkar A, Chakraborti D, Banerjee S, Das S (2005) The efficacy of a novel insecticidal protein, Allium sativum leaf lectin (ASAL), against homopteran insects monitored in transgenic tobacco. Plant Biotechnol J 3:601–611
Gatehouse AMR, Davison GM, Stewart JN, Gatehouse LN, Kumar A, Geoghegan IE, Birch ANE, Gatehouse JA (2003) Concavalin A inhibits development of tomato moth (Lacanobia oleracea) and peach-potato aphid (Myzus persicae) when expressed in transgenic potato plants. Mol Breed 5:153–165
Girard C, Rivard D, Kiggundu A, Kurnet K, Gleddie SC, Cloutier C, Michaud D (2007) A multicomponent, elicitor-inducible cystatin complex in tomato, Solanum lycopersicum. New Phytol 173:841–851
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Gutierrez-Campos R, Torres Acosta J, Saucedo-Arias LJ, Gomez-Lim MA (1999) The use of cysteine proteinase inhibitors to engineer resistance against potyvirusses in transgenic tobacco plants. Nature Biotechnol 17:1223–1226
Haq SK, Atif SM, Khan RH (2004) Protein proteinase inhibitor genes in combat against insects, pests and pathogens: natural and engineered phytoprotection. Arch Biochem Biophys 431:145–159
International Aphids Genomics Consortium (2010) Genome sequence of the pea aphid Acyrtosiphon pisum. PLoS Biology 8:e1000313
Kehr J (2006) Phloem sap proteins: their identities and potential roles in the interaction between plants and phloem-feeding insects. J Exp Bot 57:767–774
Lantz MS, Ciborowski P (1994) Zymographic techniques for detection and characterization of microbial proteases. Meth Enzymol 235:563–594
Lawo NC, Wackers FL, Romeis J (2009) Indian Bt cotton varieties do not affect the performance of cotton aphids. PLoS ONE 4:e4804
Margis R, Reis EM, Villeret V (1998) Structural and phylogenetic relationships among plant and animal cystatins. Arch Biochem Biophys 359:24–30
Martinez M, Diaz I (2008) The origin and evolution of plant cystatins and their target cysteine proteinases indicate a complex functional relationship. BMC Evol Biol 8:198
Martinez M, Lopez-Solanilla E, Rodriguez-Palenzuela P, Carbonero P, Diaz I (2003) Inhibition of plant-pathogenic fungi by the barley cystatin Hv-CPI (gene Icy) is not associated with its cysteine-proteinase inhibitory properties. Mol Plant-Microbe Interact 16:876–883
Martinez M, Diaz-Mendoza M, Carrillo L, Diaz I (2007) Carboxy terminal extended phytocystatins are bifunctional inhibitors of papain and legumain cysteine proteinases. FEBS Lett 581:2914–2918
Martinez M, Cambra I, Carrillo L, Diaz-Mendoza M, Diaz I (2009) Characterization of the entire cystatin gene family in barley and their target cathepsin L-like cysteine-proteases, partners in the hordein mobilization during seed germination. Plant Physiol 151:1531–1545
Michaud D, Cantin L, Raworth DA, Vrain TC (1996) Assessing the stability of cystatin/cysteine proteinase complexes using mildly-denaturing gelatin-polyacrylamide gel electrophoresis. Electrophoresis 17:74–79
Nissen MS, Kumar GNM, Youn B, Knowles DB, Lam KS, Ballinger WI, Knowles NR, Kang CH (2009) Characterization of Solanum tuberosum multicystatin and its structural comparison with other cystatins. Plant Cell 21:861–875
Novillo C, Castañera P, Ortego F (1997) Characterization and distribution of chymotrypsin-like and other digestive proteases in Colorado potato beetle larvae. Arch Insect Biochem Physiol 36:181–201
Oñate-Sanchez L, Vicente-Carbajosa J (2008) DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res Notes 1:93
Ortego F, Novillo C, Castañera P (1996) Characterization and distribution of digestive proteases of the stalk corn borer, Sesamia nonagrioides Lef (Lepidoptera: Noctuidae). Arch Insect Biochem Physiol 33:163–180
Pernas M, Sanchez-Monge R, Gomez L, Salcedo G (1998) A chestnut seed cystatin differentially effective against cysteine proteinase from closely related pests. Plant Mol Biol 38:1235–1242
Pernas M, Sanchez-Monge R, Sanchez-Ramos I, Lombardero M, Arteaga C, Castañera P, Salcedo G (2000) Der p1 and Der f1, the highly related and major allergens from house mites, are differentially affected by a plant cystatin. Clinic Exp Allergy 30:972–978
Porcar M, Grenier AM, Federici B, Rahbé Y (2009) Effects of Bacillus thuringiensis delta-endotoxins on the pea aphid (Acyrthosiphon pisum). Appl Environ Microbiol 75:4897–4900
Rahbé Y, Sauvion N, Febvay G, Peumans WJ, Gatehouse AMR (1995) Toxicity of lectins and processing of ingested proteins in the pea aphid Acyrthosiphon pisum. Entomol Exp Appl 76:143–155
Rahbé Y, Deraison C, Bonade-Bottino M, Girard C, Nardon C, Jouanin L (2003a) Effects of the cysteine protease inhibitor oryzacystatin (OC-I) on different aphids and reduced performance of Myzus persicae on OC-I expressing transgenic oilseed rape. Plant Sci 164:441–450
Rahbé Y, Ferrasson E, Rabesona H, Quillien L (2003b) Toxicity to the pea aphid Acyrthosiphon pisum of anti-chymotrypsin isoforms and fragments of Bowman-Birk protease inhibitors from pea seeds. Insect Biochem Mol Biol 33:299–306
Ribeiro APO, Pereira EJG, Galvan TL, Picanco MC, Picoli EAT, da Silva DJH, Fari MG, Otoni WC (2006) Effect of eggplant transformed with oryzacystatin gene on Myzus persicae and Macrosiphon euphorbiae. J Appl Entomol 130:84–90
Rispe C, Kutsukake M, Doublet V, Hudaverdian S, Legeai F, Simon J-C, Tagu D, Fukatsu T (2008) Large gene family expansion and variable selective pressures for cathepsin B in aphids. Mol Biol Evol 25:5–17
Shahidi-Noghabi S, van Damme EJM, Smagghe G (2009) Expression of Sambucus nigra agglutinin (SNA-I′) from elderberry in transgenic tobacco plants results in enhanced resistance to different insect species. Transgenic Res 18:249–259
Shahnaz SN, van Damme EJM, Smagghe G (2008) Carbohydrate-binding activity of the type-2 ribosome-inactivating protein SNA-I from elderberry (Sambucus nigra) is a determining factor for its insecticidal activity. Phytochemistry 69:2972–2978
Stubbs MT, Laber B, Bode W, Huber R, Jerala R, Lenarcic B, Turk V (1990) The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J 9:1939–1947
Taylor B, Powell A (1982) Isolation of plant DNA and RNA. Focus 4:4–6
Acknowledgments
We are grateful to Dr. Alberto Fereres (Centro de Ciencias Medioambientales, CSIC, Madrid, Spain) for providing the colonies of M. persicae. The financial support from the Spanish Ministerio de Ciencia e Innovación (project BFU2008-01166), the Agencia Española de Cooperación Internacional para el Desarrollo (project A/017236/08), the Spanish Ministerio de Medioambiente y Medio Rural y Marino, the Fund for Scientific Research (FWO-Vlaanderen, Belgium) and the Special Research Fund of Ghent University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carrillo, L., Martinez, M., Álvarez-Alfageme, F. et al. A barley cysteine-proteinase inhibitor reduces the performance of two aphid species in artificial diets and transgenic Arabidopsis plants. Transgenic Res 20, 305–319 (2011). https://doi.org/10.1007/s11248-010-9417-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-010-9417-2