Abstract
The envelope glycoprotein (E) of flavivirus is the major structural protein on the surface of the mature virions. The complexes of premembrane (prM) and E play important roles in virus assembly and fusion modulation and in potential immunity-inducing vaccines. In the present study, the cDNA encoding prM and E proteins of dengue virus type 2 (DENV-2) was subcloned into the pGAPZαA vector and further integrated into the genome of Pichia pastoris under the control of the glyceraldehyde-3-phosphate dehydrogenase (GAP) constitutive promoter. The high-level constitutive expression of recombinant E antigen was achieved in P. pastoris. Both the cell lysate and the culture supernatant, examined by electron microscopy, were found to contain DENV-2 virus-like particles (VLPs) with diameters of about 30 nm. After immunization of BALB/c mice, the VLPs exhibited similar efficacies as inactivated virus in terms of antibody induction and neutralization titer. These results suggest that recombinant DENV VLPs can be efficiently produced in the GAP promoter-based P. pastoris expression system. This system may be useful for the development of effective and economic dengue subunit vaccine.
Similar content being viewed by others
Introduction
Dengue viruses (DENVs) belong to the Flavivirus genus of the Flaviviridae family and include four serotypes (DENV-1, -2, -3, and -4) [1]. The viruses are transmitted to humans through the bite of an infected Aedes mosquito [2]. DENV causes a full spectrum of clinical manifestations, including dengue fever (DF), dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS) [2]. Annually there are an estimated 50–100 million cases of DF, and 250,000 to 500,000 cases of DHF/DSS in the world [2, 3]. However, there is still no effective commercial DENV vaccine available in spite of considerable efforts to develop live attenuated virus vaccines, inactivated whole virus vaccines and DNA vaccines [3].
The flavivirus genome consists of ~11 kb single-stranded, positive-sense RNA which encodes three structural proteins (capsid, premembrane/membrane (prM/M), and envelope (E) protein), and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [4]. Among the structural proteins, E protein is the major structural protein on the surface of the mature virions [1], and responsible for mediating viral entry functions and inducing a protective immune response [5–7]. E protein forms oligomers with prM/M protein and constitutes most of the accessible virions surface [1]. Co-expression of prM and E proteins can generate recombinant virus-like particles (VLPs) [8, 9]. Flavivirus VLPs have been shown to be similar to the infectious virions in the structural and functional features [9–11]. The processes required for VLPs production in prM/E-expressing cells, including protein synthesis, targeting the proteins to subcellular organelles, and particle release, are similar to the maturation events of authentic viruses occurring in infected cells [10, 11]. The VLPs from Tick-borne encephalitis virus (TBEV) represent an excellent model system for investigating the structural basis of viral envelope glycoprotein functions [12]. Furthermore, prior inoculation of mice with VLPs derived from flavivirus has been found to protect mice from challenge with virulent wild-type virus [9, 13]. Thus, flavivirus VLPs show considerable promise as a candidate vaccine for the prevention of flavivirus-induced diseases [13].
Several expression systems, including recombinant vaccinia viruses and mammalian cells, have been used to produce DENV VLPs as vaccine antigen [14–16]. However, because mammalian cells are susceptive to the toxicity of viral products, cell fusion and apoptosis caused by dengue virus are significant obstacles to generation of cell lines stable producing DENV VLPs [16]. Based on the previous studies of HBsAg produced in Pichia pastoris yeast [17, 18], it is feasible to overcome specific restrictions in the expression of DENV VLPs by choosing P. pastoris expression system. Sugrue et al. [19] have tried to express DENV-1 VLPs in P. pastoris by using methanol-inducible alcohol oxidase I (AOX I) promoter. However, probably because of the defect of the AOX I promoter-based system, no sequential work has been reported. Recently, a new kind of P. pastoris expression vector based on a constitutively active promoter of glyceraldehyde-3-phosphate dehydrogenase (GAP) has been used to simultaneously produce antigen [17, 18]. The GAP promoter-based expression system can enhance the yield of antigen compared to the AOX I promoter-based system, and obviate the need to use methanol during fermentation [18].
Our aim was to explore the possibility of using the GAP promoter-based P. pastoris expression system to efficiently produce DENV-2 VLPs for the development of effective and economic DENV subunit vaccine.
Materials and methods
Cells and viruses
Aedes albopictus-derived C6/36 cells were cultured at 28°C in MEM (Gibco) supplemented with 0.11% of sodium bicarbonate and 10% of fetal bovine serum (FBS) (Gibco). DENV-2 ZS 01/01strain (GeneBank accession no. EF051521) was propagated in C6/36 mosquito cell culture and used for RNA extraction, immune-electron microscopy (immune-EM), and neutralization analysis. For use in the immune-EM analysis, virus was purified by sucrose density gradient centrifugation as described previously [16].
Yeast strains, vectors, and culture media
The P. pastoris host strain was X33 (wild-type strain from Invitrogen, San Diego, CA). The expression vector pGAPZαA (Invitrogen) contains the selectable marker Zeocin, which is biofunctional in P. pastoris and Escherichia coli, the 5′ GAP promoter and the 3′ AOXTT transcription termination sequences. Pichia pastoris liquid cell cultures were grown in buffered YPD medium (1% yeast extract, 2% peptone, 2% dextrose). The YEPDS medium was YPD medium to which 18.2 g sorbitol per liter was added. To prepare plates for solid cell cultures, 2% agar (w/v) was added to the YPD medium.
Cloning of DENV-2 prM/E genes into yeast vectors
The cDNA coding the signal peptide of prM, prM, and E proteins was amplified from DENV-2 ZS 01/01 strain viral RNA by RT-PCR using the primers DY1 5′-GACTTCGAAAATGAACATCTTGAACAGGAG-3′ (Bsp119I site is underlined) and DY2 5′-GAATCTAGATCAGGCCTGCACCATGACTC-3′ (XbaI site is underlined). Synthesis of cDNA (SprM/E) by RT-PCR was carried out using Prime Script Reverse Transcriptase (TakaRa, Japan) and LATaq DNA polymerase (TakaRa, Japan) under optimized reaction conditions. The yeast vector pGAPZαA were digested with Bsp119I and XbaI. The full-length of SprM/E was cloned into the downstream of GAP promoter to generate the recombinant plasmid of pGAPZA-SprM/E. The plasmid pGAPZA-SprM/E was linearized with restriction endonuclease Bgl II and used for electroporation into P. pastoris X33. Pichia pastoris transformants were selected on YPDS medium, containing antibiotic Zeocin as described previously [20].
Expression and purification of DENV-2 virus-like particles from yeast
Positive transformants were inoculated into 10 ml of YEPD medium with Zeocin in a 250 ml flask with shaking (250 rpm) at 30°C for 24 h. These cultures were further used to inoculate 200 ml of YEPD medium in a 1000 ml flask with shaking (250 rpm) at 30°C for 120 h. The culture supernatant and cell pellet were respectively collected by centrifugation (10,000×g, 10 min, 4°C).
Cells disruption was performed using glass beads in lysis buffer (50 mM sodium phosphate, pH 7.4, 1 mM EDTA, 1 mM PMSF, and 5% glycerol). The lysate was analyzed using a 5–50% (w/v) sucrose density gradients centrifugation at 153,000×g for 6 h, at 4°C (HATICHI, P80AT rotor). The sucrose gradient was fractionated and the individual fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot. Purification of DENV-2 VLPs from the culture supernatant were precipitated by using Amicon Ultra-15 Centrifugal Filter Units with 100 kDa membrane (Millipore Corp., Billerica, MA) according to manufacturer’s instructions.
SDS-PAGE and Western blot analysis
The protein samples were separated in a 12% SDS-PAGE. For Western blot, the samples were applied to a 0.45 μm nitrocellulose membrane (Millipore Corp., Billerica, MA). The membrane was then blocked for 2 h at room temperature with phosphate-buffered saline solution (PBS) (0.1 M NaCl, 2 mM KCl, 10 mM Na2HPO4, 1 mM KH2PO4 pH 7.4) containing 5% skimmed milk, then incubated with monoclonal anti-DENV-2 E antibody (3H5 mAb, American Type Culture Collection, USA) for 1 h at 37°C. After washing, the membrane was reacted with 1:1000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Santa Cruz, CA) for 1 h at 37°C. The bands were visualized using the 3,3′ diaminobenzidine (DAB) reagent (Boster, Wuhan, China).
Determination of expression level of E antigen
A simple sandwich ELISA was developed for measuring the E antigen levels present in culture supernatant and cell lysate as described before [16]. Briefly, the 96-well microtiter plates were directly coated with samples at different time intervals (from 0 to 120 h, 50 μl/well). Purified recombinant DENV-2 E protein previously produced in P. pastoris was used as a standard [20]. The 3H5 mAb, the HRP-labeled goat anti-mouse IgG antibody (Santa Cruz, CA) and TMB substrate (Boster, Wuhan, China) were sequentially added to each well, and the optical densities (OD) were measured at 450 nm. The concentration of E antigen was calculated from absorbance values obtained with the samples and standard and was present as E antigen amount in milligram per liter (mg/l).
Immune-electron microscope analysis
DENV-2 VLPs were visualized by immune-EM analysis. The purified DENV-2 VLPs were incubated with polyclonal mouse anti-DENV-2 antibody (developed in-house) at 1:100 dilutions in PBS (pH 7.4) for 1 h at 37°C, and then incubated overnight at 4°C. After centrifugation at 23,000×g for 1 h, at 4°C, the pellets were resuspended in PBS (pH 7.4), and placed on 400-mesh carbon-coated palladium grids, then stained with 2% saturated uranyl acetate. For comparison, the purified DENV-2 virions were also visualized by immune-EM analysis. All micrographs were recorded by the Philips CM10 electron microscope operating at 40 kV.
Mice experiments
Female BALB/c mice aged 4 weeks were used in the immunization experiments. A group of 5 mice were immunized intraperitoneally (i.p.) with purified VLPs (100 μg E/mouse) with complete Freund’s adjuvant(Sigma)at a volumetric ratio of 1:1. For comparison, another group of 5 mice were immunized with the heat-inactivated DENV-2 (ZS01/01 strain) at the same dose in a similar fashion. All mice were then boosted twice in incomplete Freund’s adjuvant with the same dose at an interval of 2 weeks. The PBS immunization group was taken as the negative control. Two weeks after the last boost, serum sample were prepared and stored frozen until use.
The total anti-DENV-2 VLPs IgG in the serum samples were titrated by ELISA as described previously [21]. In brief, the purified VLPs were used as coating antigen. The plate was blocked by the blocking buffer (5% bovine serum albumin in PBS) and then washed by the wash buffer (0.05% Tween 20 in PBS). After wash, the twofold serially diluted serum samples (1/20–1/20480 dilution with the blocking buffer) were added. After 1 h incubation and another wash, the HRP-labeled goat anti-mouse IgG antibody (1:4000) was added. The titers were defined as the reciprocal of the positive highest serum dilution.
Neutralization assays
The neutralization titers were determined based on a TCID50 reduction assay in C6/36 cells [22]. In brief, the C6/36 cells were seeded to and cultured in the 96-well plates overnight until 80% confluent. Serum samples were twofold serially diluted with DMEM containing 2% FBS and mixed with equal volume of DENV-2 ZS01/01 strain (100 TCID50) at 37°C for 1 h to neutralize the infectious virus particles. The mixtures were added to the C6/36 cell culture for infection. After 7-day culture, the neutralization titers were read as the highest dilutions that could result in a 50% reduction in the cytopathic effect (CPE).
Results
Generation of recombinant Pichia clones expressing DENV-2 VLPs
GAP promoter-driven constitutive expression of DENV-2 prM/E was achieved by integrating a linearized pGAPZA-SprM/E plasmid into the X33 wild-type P. pastoris genome at the GAP locus (Fig. 1). Pichia pastoris X33 transformants generated from electroporation experiments with pGAPZA-SprM/E were selected on YPDS plates containing Zeocin incubated at 30°C for 3 days. The presence of SprM/E insert in these Zeo-resistant transformants was checked by PCR.
Construction of recombinant plasmid pGAPZA-SprM/E. The cDNA coding the signal sequence of prM sequences (corresponding to amino acid residues 90–110 in the 3′ region of C), prM and E genes was amplified from DENV-2 ZS 01/01 strain viral RNA by RT-PCR using the primers DY1 and DY2. the SprM/E gene was then inserted, in the sense orientation, into the unique Bsp119I and XbaI sites of pGAPZα A plasmid to create the recombinant pGAPZA-SprM/E expression vector. S the signal peptide of prM, prM premembrane protein, E envelope protein
Analysis of continuously production of DENV-2 E antigen in P. pastoris
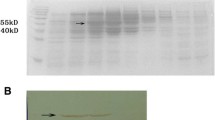
PCR-positive Pichia clones were grown separately in YPD medium with Zeocin for 120 h. To characterize the continuous expression of VLPs in this constitutive expression system, E antigen at different time intervals were assayed by quantitative ELISA and Western blot using 3H5 mAb (Fig. 2). Figure 2a shows E antigen production and cell growth data of the constitutive clone (pGAPZA-SprM/E-X33) in a continuous mode. The cell density increased rapidly, and reached steady state on day 2 of continuous mode. Similarly, the concentration of the extracellular E antigen also increased rapidly, reached about 100 mg/l within 48 h. Compared with the rapid enhancement of E antigen in the culture supernatant, the expression level of the intracellular E antigen was maintained relatively stable from 12 to 120 h, at an average amount of about 400 mg/l. The results of immunoblot indicated that a single E antigen band with a molecular weight of about 52 kDa was observed both in the culture supernatant and the cell lysate (Fig. 2b).
Assay of the continuous expression of DENV-2 E antigen. a At various times after the cell growth was initiated, samples from the culture supernatant and the cell lysate were tested to determine the expression levels of E by performing ELISA, as well as to monitor the cell growth densities. b Samples from the culture supernatant and the cell lysate were applied for Western blot analysis. The primary antibodies for ELISA and Western blot are the 3H5 mAb. The size of molecular weight marker is shown in kDa
Purification of yeast-derived DENV-2 VLPs and immune-EM analysis
The lysate of yeast cell expressing DENV-2 VLPs were analyzed using a 5–50% sucrose density gradient centrifugation. After centrifugation, each sucrose fraction was collected and assayed for the presence of DENV-2 VLPs by Western blot using 3H5 mAb. The immunoblot evidenced that E antigen was present in the fractions 7 to 10, which correspond to a buoyant density of about 1.14 g/cm3 (Fig. 3).
Sucrose gradient sedimentation analysis. The sucrose fractions were collected by upward displacement and analyzed for the presence of E protein by SDS-PAGE and Western blot using the 3H5 mAb. Twelve of the 20 collected fractions are shown; E protein was not detected in the other eight fractions. Arrow heads indicate E antigen. The size of molecular weight marker is shown in kDa
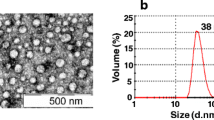
The purified samples were analyzed by immune-EM to characterize DENV-2 VLPs. The purified samples were incubated with polyclonal mouse anti-DENV-2 antibody for 1 h at 37°C, and then incubated overnight at 4°C. After centrifugation, the pellets were resuspended and examined by EM. The spherical particles with diameters of about 30 nm were detected by immune-EM (Fig. 4a, b). In addition, several larger particles with diameters of about 55 nm were also observed (Fig. 4e), but the proportion of the 30 nm particles was much greater than the 55 nm particles. The similar situation was also observed in the purified DENV-2 virions (Fig. 4c, d). The small particles were consistent with the size of the flavivirus subviral particles [10, 12], and the large particles had approximately the same size as the flavivirus mature virions [1, 23, 24]. These results demonstrated that the recombinant DENV-2 VLPs expressed by P. pastoris can be identified by anti-DENV-2 sera, and the recombinant VLPs exhibited the similar structural and physicochemical features to the virions produced in the infected cells. It suggests that the P. pastoris expression system can provide proper processing environments for dengue viral proteins and the assembly of VLPs.
Immunogenicity of DENV-2 VLPs
The purified DENV-2 VLPs were evaluated for their ability to elicit the specific antibodies response in mice. Female BALB/c mice were immunized i.p. with purified VLPs (100 μg E/mouse) and received booster injections. For comparison, the mice were immunized with heat-inactivated DENV-2 virus at the same dose in a similar fashion. The anti-DENV-2 VLPs antibody titration showed that immunization with VLPs elicited the specific antibody responses in BABL/c mice. The antibody titers reached above 1:10,000 after the booster immunization (Fig. 5). The similar antibody titer was also detected in the mice immunized with heat-inactivated DENV-2. In contrast, PBS group were all negative. Meanwhile, the ability of antibodies to neutralize the live DENV-2 virus was examined by neutralization assays. The serum samples were twofold serially diluted and mixed with infectious DENV-2 virus ZS01/01strain (100TCID50), and the titers were read as the highest dilution that resulted in a 50% reduction in CPE. The results indicated that the neutralization titers induced by the PBS were barely detectable (<1:10). The VLPs resulted in a neutralization titer similar to that of the heat-inactivated DENV-2 whole virus, with a neutralization titer of 1:45 after the booster immunization (Table 1).
Discussion
In the present article, we demonstrated that the expression of DENV-2 prM and E proteins by using GAP promoter in P. pastoris resulted in the efficient production of VLPs. Meanwhile, the recombinant VLPs were excellent immunogens, and exhibited similar efficacies as inactivated whole virus in eliciting neutralizing antibodies. To develop noninfectious DENV VLPs as the potential source of a biosynthetic subunit vaccine, it is necessary to establish a safe and stably expression system for continuously producing DENV VLPs [16]. However, previous efforts on the exploration of establishing mammalian cell lines for DENV VLPs have been impaired by multiple blocks to the VLPs production [16]. To overcome specific restrictions to the expression of DENV VLPs, we adopted a well-tolerated P. pastoris yeast cells to produce DENV-2 VLPs. Meanwhile, P. pastoris is one of the most economic and convenient expression systems to produce large amount of antigens for vaccine development [25, 26]. It has no pathogenic relationship with man, is free of endotoxin and has been used in industrial fermentations for centuries [20, 21]. The GAP promoter has been successfully applied for high-level production of HBsAg in yeast expression system [17, 18]. In addition, the signal peptides of prM were also introduced to the downstream of GAP promoter to improve the secretion of DENV VLPs. Our results indicated that DENV-2 VLPs were high level and continuously produced in this constitutive expression system. Moreover, the VLPs were efficiently secreted into the culture supernatant. It indicated that the GAP promoter-based P. pastoris expression system are suitable for the large amount of DENV VLPs production, and the signal peptide of prM can be recognized by the yeast secretory apparatus and lead the secretion of DENV-2 VLPs.
So far, the recombinant E proteins produced in several expression systems have been developed as subunit vaccine candidates for dengue [26–29]. Nevertheless, several lines of evidence indicate that a recombinant plasmid with expressing flavivirus prM and E was more immunogenic in mice than that expressing E alone [13, 30]. Furthermore, comparative studies of the soluble form and the particulate form of E from TBEV have demonstrated that only VLPs induces the high titers of neutralizing antibody and protection in mice [13]. In the present study, we found that DENV-2 VLPs could induce strong antibody responses in immunized mice. Meanwhile, the antibodies induced by VLPs were able to neutralize the live DENV-2 virus at a neutralizing titer of 1:45. DENV-2 VLPs exhibit similar efficacies as inactivated whole virus in terms of antibody induction and neutralizing titer.
In conclusion, the generation of DENV-2 VLPs from the GAP promoter-based P. pastoris expression system suggests the feasibility of establishing efficiently and constitutively producing DENV VLPs. The present study provides a suitable method for developing subunit dengue vaccine for the prevention of dengue virus infection.
References
R.J. Kuhn, W. Zhang, M.G. Rossmann, S.V. Pletnev, J. Cover, E. Lenches, C.T. Jones, S. Mukhopadhyay, P.R. Chipman, E.G. Strauss, T.S. Baker, J.H. Strauss, Cell 108, 717–725 (2002)
S.B. Halstead, Lancet 370, 1644–16523 (2007)
S.S. Whitehead, J.E. Blaney, A.P. Durbin, B.R. Murphy, Nat. Rev. Microbiol. 5, 518–528 (2007)
B.D. Lindenbach, C.R. Rice, Adv. Virus Res. 59, 23–61 (2003)
M.W. Chiu, Y.L. Yang, Biochem. Biophys. Res. Commun. 309, 672–678 (2003)
S. Khanam, B. Etemad, N. Khanna, S. Swaminathan, Am. J. Trop. Med. Hyg. 74, 266–277 (2006)
K. Raviprakash, T.J. Kochel, D. Ewing, M. Simmons, I. Phillips, C.G. Hayes, K.R. Porter, Vaccine 18, 2426–2434 (2000)
R. Gehrke, M. Ecker, S.W. Aberle, S.L. Allison, F.X. Heinz, C.W. Mandl, J. Virol. 77, 8924–8933 (2003)
E. Konishi, A. Fujii, P.W. Mason, J. Virol. 75, 2204–2212 (2001)
I. Ferlenghi, M. Clarke, T. Ruttan, S.L. Allison, J. Schalich, F.X. Heinz, S.C. Harrison, F.A. Rey, S.D. Fuller, Mol. Cell 7, 593–602 (2001)
I.C. Lorenz, J. Kartenbeck, A. Mezzacasa, S.L. Allison, F.X. Heinz, A. Helenius, J. Virol. 77, 4370–4382 (2003)
J. Schalich, S.L. Allison, K. Stiasny, C.W. Mandl, C. Kunz, F.X. Heinz, J. Virol. 70, 4549–4557 (1996)
F.X. Heinz, S.L. Allison, K. Stiasny, J. Schalich, H. Holzmann, C.W. Mandl, C. Kunz, Vaccine 13, 1636–1642 (1995)
B.A. Fonseca, S. Pincus, R.E. Shope, E. Paoletti, P.W. Mason, Vaccine 12, 279–285 (1994)
D.E. Purdy, G.J. Chang, Virology 333, 239–250 (2005)
E. Konishi, A. Fujii, Vaccine 20, 1058–1067 (2002)
G.A. Bitter, K.M. Egan, Gene 32, 263–274 (1984)
A. Vassileva, D.A. Chugh, S. Swaminathan, N. Khanna, J. Biotechnol. 88, 21–35 (2001)
R.J. Sugrue, J. Fu, J. Howe, Y.C. Chan, J. Gen. Virol. 78, 1861–1866 (1997)
H.Y. Wei, L.F. Jiang, Y.H. Xue, D.Y. Fang, H.Y. Guo, J. Virol. Methods 109, 17–23 (2003)
H. Bisht, D.A. Chugh, M. Raje, S.S. Swaminathan, N. Khanna, J. Biotechnol. 99, 97–110 (2002)
S.P. Chen, M. Yu, T. Jiang, Y.Q. Deng, C.F. Qin, E.D. Qin, DNA Cell Biol. 26, 361–367 (2007)
Y. Modis, S. Ogata, D. Clements, S.C. Harrison, Nature 427, 313–319 (2004)
S.L. Allison, Y.J. Tao, G. O’Riordain, C.W. Mandl, S.C. Harrison, F.X. Heinz, J. Virol. 77, 11357–11366 (2003)
C.P. Chuck, C.H. Wong, L.M. Chow, K.P. Fung, M.M. Waye, S.K. Tsui, Virus Genes 38, 1–9 (2009)
R.J. Sugrue, T. Cui, Q. Xu, J. Fu, Y.C. Chan, J. Virol. Methods 69, 159–169 (1997)
R. Men, M. Bray, C. Lai, J.Virol. 65, 1400–1407 (1991)
J.P. Babu, P. Pattnaik, N. Gupta, A. Shrivastava, M. Khan, P.V. Rao, Vaccine 26, 4655–4663 (2008)
E. Konishi, M. Yamaoka, I. Kurane, P.W. Mason, Vaccine 18, 1133–1139 (2000)
E. Konishi, K.S. Win, I. Kurane, P.W. Mason, R.E. Shope, F.A. Ennis, Vaccine 15, 281–286 (1997)
Acknowledgments
This work was jointly funded by the National Science Foundation (no. U0632002) and National High Technology Research Development Program (no. 2006AA02A223). We thank Dr. Xi Huang and Dr. Jishen Wen for critical reading of the manuscript and for many helpful suggestions, and thank Dr. Wanying Yang for kindly providing the vector.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, W., Jiang, H., Zhou, J. et al. Recombinant dengue virus-like particles from Pichia pastoris: efficient production and immunological properties. Virus Genes 40, 53–59 (2010). https://doi.org/10.1007/s11262-009-0418-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-009-0418-2