Abstract
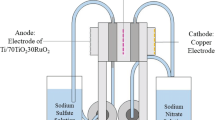
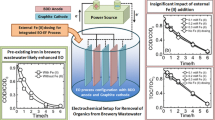
Nitrates in concentrated brines can be electrochemically reduced in the cathodic chamber of a split-cell electrochemical reactor with formation of ammonium (and small amounts of nitrite). Fortunately, ammonium may be electrochemically oxidized to nitrogen gas in the anodic reaction chamber if a coupled sequential process is used. The presence of chloride in the brine waste is an important consideration in oxidative electrochemical processes, however, because it cycles through oxidized and reduced states at the electrode surfaces and in the bulk solution. Electrochemical oxidation converts chloride ions to “active chlorine” species with additional oxidizing capability (chlorine, hypochlorous acid and hypochlorite – essentially bleach), as well as to chlorates, depending on the reaction conditions. The production of these active species improves treatment performance in the ammonium oxidation phase since oxidation is no longer limited to the electrode surface. However, the process must be engineered to minimize loss of process efficiency due to parasitic side reactions (chloramines and chlorate). In this study, two-stage batch electrolysis was conducted using a three-electrode (copper anode, platinum-coated titanium cathode, silver/silver chloride reference) electrochemical cell, with the anodic and cathodic chambers separated by a Nafion 117 membrane. Treatment of nitrate and ammonium was tested with and without the presence of chloride in the waste. No significant difference was observed in cathodic nitrate reduction with chloride present or absent. However, the presence of chloride in the solution favored overall soluble nitrogen elimination upon oxidation. Increasing applied current increased production of undesirable byproducts (especially chlorate).






Similar content being viewed by others
References
Bae, B-., Jung, Y-., Han, W-., & Shin, H-. (2000). Improved brine recycling during nitrate removal using ion exchange. Water Research, 36(13), 3330–3340.
Bohdziewicz, J., Bodzek, M., & Wasik, E. (1999). The application of reverse osmosis and nanofiltration to the removal of nitrates from groundwater. Desalination, 121(2), 139–147.
Chiang, L-., Chang, J-., & Wen, T-. (1995). Indirect oxidation effect in electrochemical oxidation treatment of landfill leachate. Water Research, 29(2), 671–678.
Cossu, R., Polcaro, A. M., Lavagnolo, M. C., Mascia, M., Palmas, S., & Renoldi, F. (1998). Electrochemical treatment of landfill leachate: Oxidation at Ti/PbO2 and Ti/SnO2 anodes. Environmental Science and Technology, 32(22), 3570–3573.
Czarnetzki, L. R., & Janssen, L. J. J. (1992). Formation of hypochlorite, chlorate and oxygen during NaCl electrolysis from alkaline solutions at an RuO2/TiO2 anode. Journal of Applied Electrochemistry, 22(4), 315–324.
Fóti, G., Mousty, C., Novy, K., Comninellis, C., & Reid, V. (2000). Pt/Ti electrode preparation methods: Application to the electrooxidation of isopropanol. Journal of Applied Electrochemistry, 30(2), 147–151.
Gootzen, J. F. E., Lefferts, L., & Van Veen, J. A. R. (1999). Electrocatalytic nitrate reduction on palladium based catalysts activated with germanium. Applied Catalysis A: General, 188(1–2), 127–136.
Graillon, S., Persin, F., Pourcelly, G., & Gavach, C. (1996). Development of electrodialysis with bipolar membrane for the treatment of concentrated nitrate effluents. Desalination, 107(2), 159–169.
Kim, K-., Kim, Y-., Kim, I-., Park, G-., & Lee, E-. (2006). Characteristics of mediated enzymatic nitrate reduction by gallocyanine-bound nanoporous electrode. Water Research, 40(7), 1431–1441.
Paidar, M., Bouzek, K., Jelínek, L., & Matějka, Z. (2004). A combination of ion exchange and electrochemical reduction for nitrate removal from drinking water: Part II: Electrochemical treatment of a spent regenerant solution. Water Environment Research, 76(7), 2691–2698.
Paidar, M., Roušar, I., & Bouzek, K. (1999). Electrochemical removal of nitrate ions in waste solutions after regeneration of ion exchange columns. Journal of Applied Electrochemistry, 29(5), 611–617.
Polatides, C., Dortsiou, M., & Kyriacou, G. (2005). Electrochemical removal of nitrate ion from aqueous solution by pulsing potential electrolysis. Electrochimica Acta, 50(25–26), 5237–5241.
Szpyrkowicz, L., Daniele, S., Radaelli, M., & Specchia, S. (2006). Removal of NO3 − from water by electrochemical reduction in different reactor configurations. Applied Catalysis B, Environmental, 66(1–2), 40–50.
Szpyrkowicz, L., Kaul, S. N., Neti, R. N., & Satyanarayan, S. (2005). Influence of anode material on electrochemical oxidation for the treatment of tannery wastewater. Water Research, 39(8), 1601–1613.
Tada, K., Kawaguchi, T., & Shimazu, K. (2004). High electrocatalytic performance of Pd/Sn/Au electrodes for nitrate reduction. Journal of Electroanalytical Chemistry, 572(1), 93–99.
Wang, Y., Qu, J. H., & Liu, H. J. (2006). Preparation and electrochemical properties of the Pd-modified Cu electrode for nitrate reduction in water. Chinese Chemical Letters, 17(1), 61–64.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, J., Kupferle, M.J. Two-stage Sequential Electrochemical Treatment of Nitrate Brine Wastes. Water Air Soil Pollut: Focus 8, 379–385 (2008). https://doi.org/10.1007/s11267-007-9153-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11267-007-9153-7