Abstract
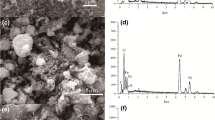
A magnetic chitosan-modified Fe3O4@SiO2 with sodium tripolyphosphate adsorbent (MTPCS) was synthesized by surface modification of Fe3O4@SiO2 with chitosan using sodium tripolyphosphate (STPP) as the cross-linker in buffer solution for the adsorption of Cu(II) ions from aqueous solution. The structure and morphology of this magnetic nanoadsorbent were examined by powder X-ray diffraction (XRD), transmission electron microscopy (TEM), BET surface area measurements, Fourier transform infrared spectrometer (FTIR), and X-ray photoelectron spectroscopy (XPS). The effects of initial pH, adsorbent amount, and initial concentration of heavy metal ions were investigated by batch experiments. Moreover, adsorption isotherms, kinetics, and thermodynamics were studied to understand the mechanism of adsorbing metal ions by synthesized MTPCS. The results revealed that adsorption kinetics was best depicted by the pseudo-second-order rate mode and intraparticle-diffusion models. The adsorption isotherm fitted well to the Langmuir model. Moreover, thermodynamic study verified the adsorption process was endothermic and spontaneous in nature. The maximum adsorption occurred at pH 5 ± 0.1, and the adsorbent could be used as a reusable adsorbent with convenient conditions.












Similar content being viewed by others
References
Abbas, A., Al-Amer, A. M., Laoui, T., Al-Marri, M. J., Nasser, M. S., Khraisheh, M., & Atieh, M. A. (2016). Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Separation and Purification Technology, 157, 141–161.
Ali, I. (2012). New generation adsorbents for water treatment. Chemical Reviews, 112(10), 5073–5091.
Arous, O., Gherrou, A., & Kerdjoudj, H. (2004). Removal of Ag (Ӏ), Cu (II) and Zn (ӀӀӀ) ions with a supported liquid membrane containing cryptands as carriers. Desalination, 161(3), 295–303.
Banerjee, S. S., & Chen, D. H. (2007). Fast removal of copper ions by gum arabic modified magnetic nano-adsorbent. Journal of Hazardous Materials, 147(3), 792–799.
Chan, P. Y., Ng, S. L., Seng, C. E., & Lim, P. E. (2012). Removal of Cu(II) from aqueous solutions by living and non-living cultured sludges: equilibrium modelling. International Journal of Environmental Technology and Management, 15(1), 79–93.
Chang, Y. C., & Chen, D. H. (2005). Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of cu(II) ions. Journal of Colloid and Interface Science, 283(2), 446–451.
Demiral, H., & Güngör, C. (2016). Adsorption of copper (II) from aqueous solutions on activated carbon prepared from grape bagasse. Journal of Cleaner Production, 124, 103–113.
Deng, H., Li, X., Peng, Q., Wang, X., Chen, J., & Li, Y. (2005). Monodisperse magnetic single-crystal ferrite microspheres. Angewandte Chemie, 117(18), 2842–2845.
Dib, A., & Makhloufi, L. (2004). Cementation treatment of copper in wastewater: mass transfer in a fixed bed of iron spheres. Chemical Engineering and Processing: Process Intensification, 43(10), 1265–1273.
Doğan, M., Türkyilmaz, A., Alkan, M., & Demirbaş, Ö. (2009). Adsorption of copper (II) ions onto sepiolite and electrokinetic properties. Desalination, 238(1–3), 257–270.
Donia, A. M., Atia, A. A., & Elwakeel, K. Z. (2008). Selective separation of mercury (II) using magnetic chitosan resin modified with Schiff’s base derived from thiourea and glutaraldehyde. Journal of Hazardous Materials, 151(2), 372–379.
España, J. S., Pamo, E. L., Pastor, E. S., Andrés, J. R., & Rubí, J. A. M. (2006). The removal of dissolved metals by hydroxysulphate precipitates during oxidation and neutralization of acid mine waters. Iberian Pyrite Belt. Aquatic Geochemistry, 12(3), 269–298.
da Fonseca, M. G., de Oliveira, M. M., Arakaki, L. N., Espinola, J. G., & Airoldi, C. (2005). Natural vermiculite as an exchanger support for heavy cations in aqueous solution. Journal of Colloid and Interface Science, 285(1), 50–55.
Guibal, E. (2004). Interactions of metal ions with chitosan-based sorbents: a review. Separation and Purification Technology, 38(1), 43–74.
Hao, Y. M., Man, C., & Hu, Z. B. (2010). Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. Journal of Hazardous Materials, 184(1), 392–399.
Hasan, S., Ghosh, T. K., Viswanath, D. S., & Boddu, V. M. (2008). Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. Journal of Hazardous Materials, 152(2), 826–837.
Hsieh, S. H., Horng, J. J., & Tsai, C. K. (2006). Growth of carbon nanotube on micro-sized Al2O3 particle and its application to adsorption of metal ions. Journal of Materials Research, 21(5), 1269–1273.
Li, N., & Bai, R. (2005). Copper adsorption on chitosan-cellulose hydrogel beads: behaviors and mechanisms. Separation and Purification Technology, 42(3), 237–247.
Lin, L. C., Li, J. K., & Juang, R. S. (2008). Removal of Cu (II) and Ni (II) from aqueous solutions using batch and fixed-bed ion exchange processes. Desalination, 225(1–3), 249–259.
Liu, Y., Man, C., & Hao, Y. (2013). Study on the adsorption of Cu(II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chemical Engineering Journal, 218(218), 46–54.
Mi, F. L., Shyu, S. S., Lee, S. T., & Wong, T. B. (1999). Kinetic study of chitosan-tripolyphosphate complex reaction and acid-resistive properties of the chitosan-tripolyphosphate gel beads prepared by in-liquid curing method. Journal of Polymer Science Part B: Polymer Physics, 37(14), 1551–1564.
Mi, F. L., Sung, H. W., Shyu, S. S., Su, C. C., & Peng, C. K. (2003). Synthesis and characterization of biodegradable TPP/genipin co-crosslinked chitosan gel beads. Polymer, 44(21), 6521–6530.
Monier, M., Ayad, D. M., Wei, Y., & Sarhan, A. A. (2010). Preparation and characterization of magnetic chelating resin based on chitosan for adsorption of Cu (II) ions, Co (II) ions, and Ni (II) ions. Reactive and Functional Polymers, 70(4), 257–266.
Mubarak, A. A., El-Shazly, A. H., & Konsowa, A. H. (2004). Recovery of copper from industrial waste solution by cementation on reciprocating horizontal perforated zinc disc. Desalination, 167, 127–133.
Öğütveren, Ü. B., Koparal, S., & Özel, E. (1997). Electrodialysis for the removal of copper ions from wastewater. Journal of Environmental Science & Health Part A, 32(3), 749–761.
Özcan, A., Öncü, E. M., & Özcan, A. S. (2006). Kinetics, isotherm and thermodynamic studies of adsorption of acid blue 193 from aqueous solutions onto natural sepiolite. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 277(1), 90–97.
Ozmen, M., Can, K., Arslan, G., Tor, A., Cengeloglu, Y., & Ersoz, M. (2010). Adsorption of Cu(II) from aqueous solution by using modified Fe3O4 magnetic nanoparticles. Desalination, 254(1–3), 162–169.
Pan, B., Pan, B., Zhang, W., Lv, L., Zhang, Q., & Zheng, S. (2009). Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chemical Engineering Journal, 151(1), 19–29.
Rangsivek, R., & Jekel, M. R. (2005). Removal of dissolved metals by zero-valent iron (ZVI): Kinetics, equilibria, processes and implications for stormwater runoff treatment. Water Research, 39(17), 4153–4163.
Ren, Y. M., Wei, X. Z., & Zhang, M. L. (2008). Adsorption character for removal Cu(II) by magnetic Cu(II) ion imprinted composite adsorbent. Journal of Hazardous Materials, 158(1), 14–22.
Ren, Y., Abbood, H. A., He, F., Peng, H., & Huang, K. (2013). Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chemical Engineering Journal, 226, 300–311.
Repo, E., Warchol, J. K., Kurniawan, T. A., & Sillanpää, M. E. (2010). Adsorption of Co (II) and Ni (II) by EDTA-and/or DTPA-modified chitosan: kinetic and equilibrium modeling. Chemical Engineering Journal, 161(1), 73–82.
Robert, C. O., & Handley, O. (2000). Modern magnetic materials: principles and applications. New York: John Wiley and Sons.
Shukla, S. R., Gaikar, V. G., Pai, R. S., & Suryavanshi, U. S. (2009). Batch and column adsorption of Cu (II) on unmodified and oxidized coir. Separation Science and Technology, 44(1), 40–62.
Sun, S., & Wang, A. (2006). Adsorption properties of carboxymethyl-chitosan and cross-linked carboxymethyl-chitosan resin with Cu (II) as template. Separation and Purification Technology, 49(3), 197–204.
Türkmen, D., Yılmaz, E., Öztürk, N., Akgöl, S., & Denizli, A. (2009). Poly (hydroxyethyl methacrylate) nanobeads containing imidazole groups for removal of Cu (II) ions. Materials Science and Engineering: C, 29(6), 2072–2078.
Wan Ngah, W. S., & Fatinathan, S. (2008). Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chemical Engineering Journal, 143(1–3), 62–72.
Wan Ngah, W. S., & Fatinathan, S. (2010). Adsorption characterization of Pb (II) and Cu (II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. Journal of Environmental Management, 91(4), 958–969.
Wan Ngah, W. S., Endud, C. S., & Mayanar, R. (2002). Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. Reactive and Functional Polymers, 50(2), 181–190.
Wang, S., Li, L., & Zhu, Z. H. (2007). Solid-state conversion of fly ash to effective adsorbents for Cu removal from wastewater. Journal of Hazardous Materials, 139(2), 254–259.
Wang, J., Zheng, S., Shao, Y., Liu, J., Xu, Z., & Zhu, D. (2010). Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. Journal of Colloid and Interface Science, 349(1), 293–299.
Wang, X. S., Zhu, L., & Lu, H. J. (2011). Surface chemical properties and adsorption of Cu(II) on nanoscale magnetite in aqueous solutions. Desalination, 276(1), 154–160.
Wu, F. C., Tseng, R. L., & Juang, R. S. (2010). A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. Journal of Environmental Management, 91(4), 798–806.
Yavuz, M., Gode, F., Pehlivan, E., Ozmert, S., & Sharma, Y. C. (2008). An economic removal of Cu2+ and Cr3+ on the new adsorbents: pumice and polyacrylonitrile/pumice composite. Chemical Engineering Journal, 137(3), 453–461.
Yin, P., Xu, Q., Qu, R., & Zhao, G. (2009). Removal of transition metal ions from aqueous solutions by adsorption onto a novel silica gel matrix composite adsorbent. Journal of Hazardous Materials, 169(1), 228–232.
Zhang, X., & Bai, R. (2003). Mechanisms and kinetics of humic acid adsorption onto chitosan-coated granules. Journal of Colloid and Interface Science, 264(1), 30–38.
Zhang, Y. X., Yu, X. Y., Jin, Z., Jia, Y., Xu, W. H., Luo, T., Zhu, B. J., Liu, J. H., & Huang, X. J. (2011). Ultra high adsorption capacity of fried egg jellyfish-like γ-AlOOH (Boehmite)@ SiO2/Fe3O4 porous magnetic microspheres for aqueous Pb (II) removal. Journal of Materials Chemistry, 21(41), 16550–16557.
Zhou, Y. T., Nie, H. L., Branford-White, C., He, Z. Y., & Zhu, L. M. (2009a). Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. Journal of Colloid and Interface Science, 330(1), 29–37.
Zhou, L. C., Li, Y. F., Bai, X., & Zhao, G. H. (2009b). Use of microorganisms immobilized on composite polyurethane foam to remove Cu (II) from aqueous solution. Journal of Hazardous Materials, 167(1), 1106–1113.
Acknowledgments
This work was jointly supported by the National Natural Science Foundation of China (Nos. 21367016, 51104073, 51408282), the Science Research Fund in Yunnan Province Department of Education (No.2015 J028), and Key Fund Project of Yunnan Provincial Department of Education (No.2015Z044).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, X., Li, K., Ning, P. et al. Removal of Cu(II) Ions from Aqueous Solution by Magnetic Chitosan-Tripolyphosphate Modified Silica-Coated Adsorbent: Characterization and Mechanisms. Water Air Soil Pollut 228, 302 (2017). https://doi.org/10.1007/s11270-017-3482-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3482-6