Abstract
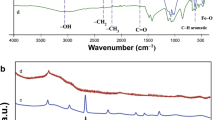
Spherical biochar derived from saccharides (glucose, sucrose, and xylose) was prepared through two steps: pre-hydrothermal carbonization at 190 °C and calcination at low temperatures (200–325 °C). The spherical biochar was characterized by Brunauer–Emmett–Teller (BET) surface area analysis, Fourier transform infrared spectroscopy, zeta potential, scanning and transmission electron microscopies, and X-ray diffraction. The result indicated that the spherical biochar exhibited low S BET (15–22 m2/g), but abundant superficial active oxygen-containing functional groups. The spherical biochar possessed a negatively charged surface within solution pH 2.0–11. The adsorption process of Pb2+, Cu2+, and methylene green 5 (MG5) was strongly dependent on the solution pH and reached fast equilibrium at approximately 60 min. The maximum Langmuir adsorption capacity (Q°max) exhibited the following order: glucose-biochar > sucrose-biochar > xylose-biochar prepared at 300 °C. The selective adsorption order of glucose-biochar was Cu2+ (0.894 mmol/g) > Pb2+ (0.848 mmol/g) > MG5 (0.334 mmol/g). The electrostatic attraction played a determining role in the adsorption mechanism of pollutant cations. The adsorption of anionic dye (acid red 1) on the spherical biochar was negligible because of electrostatic repulsion. The spherical biochar can serve as a newer and promising adsorbent to remove toxic pollutant cations from water media.







Similar content being viewed by others
References
Anastopoulos, I., & Kyzas, G. Z. (2016). Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena? Journal of Molecular Liquids, 218, 174–185.
Blackburn, R. S. (2004). Natural polysaccharides and their interactions with dye molecules: applications in effluent treatment. Environmental Science & Technology, 38(18), 4905–4909.
Blanchard, G., Maunaye, M., & Martin, G. (1984). Removal of heavy metals from waters by means of natural zeolites. Water Research, 18(12), 1501–1507.
Chen, B., Zhou, D., & Zhu, L. (2008). Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environmental Science & Technology, 42(14), 5137–5143.
Chen, X., Chen, G., Chen, L., Chen, Y., Lehmann, J., McBride, M. B., & Hay, A. G. (2011). Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresource Technology, 102(19), 8877–8884.
Coughlin, R. W., & Ezra, F. S. (1968). Role of surface acidity in the adsorption of organic pollutants on the surface of carbon. Environmental Science & Technology, 2(4), 291–297.
Crombie, K., Mašek, O., Sohi, S. P., Brownsort, P., & Cross, A. (2013). The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy, 5(2), 122–131.
Figueiredo, J. L., Pereira, M. F. R., Freitas, M. M. A., & Órfão, J. J. M. (1999). Modification of the surface chemistry of activated carbons. Carbon, 37(9), 1379–1389.
Freedonia (2016). World activated carbon. Industry Studies and Freedonia Focus Report.
Gong, Y., Wang, H., Wei, Z., Xie, L., & Wang, Y. (2014). An efficient way to introduce hierarchical structure into biomass-based hydrothermal carbonaceous materials. ACS Sustainable Chemistry & Engineering, 2(10), 2435–2441.
Härmas, M., Thomberg, T., Kurig, H., Romann, T., Jänes, A., & Lust, E. (2016). Microporous–mesoporous carbons for energy storage synthesized by activation of carbonaceous material by zinc chloride, potassium hydroxide or mixture of them. Journal of Power Sources, 326, 624–634.
Inada, M., Enomoto, N., Hojo, J., & Hayashi, K. (2017). Structural analysis and capacitive properties of carbon spheres prepared by hydrothermal carbonization. Advanced Powder Technology, 28(3), 884–889.
Jain, A., Balasubramanian, R., & Srinivasan, M. P. (2016). Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chemical Engineering Journal, 283, 789–805.
Jirka, S. & Tomlinson, T. (2015). State of the biochar industry. A survey of commercial activity in the biochar field. International biochar initiative.
Lagergren, S. (1898). About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar, 24(4), 1–39.
Li, D.-C., & Jiang, H. (2017). The thermochemical conversion of non-lignocellulosic biomass to form biochar: a review on characterizations and mechanism elucidation. Bioresource Technology. https://doi.org/10.1016/j.biortech.2017.07.029.
Li, J., Dai, J., Liu, G., Zhang, H., Gao, Z., Fu, J., He, Y., & Huang, Y. (2016). Biochar from microwave pyrolysis of biomass: a review. Biomass and Bioenergy, 94, 228–244.
Mattson, J. A., Mark, H. B., Malbin, M. D., Weber, W. J., & Crittenden, J. C. (1969). Surface chemistry of active carbon: specific adsorption of phenols. Journal of Colloid and Interface Science, 31(1), 116–130.
Moreno-Castilla, C. (2004). Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon, 42(1), 83–94.
Pellera, F.-M., Giannis, A., Kalderis, D., Anastasiadou, K., Stegmann, R., Wang, J.-Y., & Gidarakos, E. (2012). Adsorption of Cu(II) ions from aqueous solutions on biochars prepared from agricultural by-products. Journal of Environmental Management, 96(1), 35–42.
Roginsky, S., & Zeldovich, Y. B. (1934). The catalytic oxidation of carbon monoxide on manganese dioxide. Acta Physics Chemistry USSR, 1, 554.
Romero-Anaya, A. J., Ouzzine, M., Lillo-Ródenas, M. A., & Linares-Solano, A. (2014). Spherical carbons: synthesis, characterization and activation processes. Carbon, 68, 296–307.
Sevilla, M., Fuertes, A. B., & Mokaya, R. (2011). High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy & Environmental Science, 4(4), 1400–1410.
Siegel, F. R. (2002). Contaminant/natural background values: timing and processes. Environmental geochemistry of potentially toxic metals, Springer, pp. 77–101.
Singh, B., Camps-Arbestain, M., & Lehmann, J. (2017). Biochar: a guide to analytical methods. Clayton: CSIRO Publishing.
Tran, H. N., You, S.-J., & Chao, H.-P. (2016). Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: a comparison study. Journal of Environmental Chemical Engineering, 4(3), 2671–2682.
Tran, H. N., Huang, F.-C., Lee, C.-K., & Chao, H.-P. (2017a). Activated carbon derived from spherical hydrochar functionalized with triethylenetetramine: synthesis, characterizations, and adsorption application. Green Processing and Synthesis. https://doi.org/10.1515/gps-2016-0178.
Tran, H. N., C.-K. Lee, T. V. Nguyen, & Chao, H.-P. (2017b). Saccharide-derived microporous spherical biochar prepared from hydrothermal carbonization and different pyrolysis temperatures: synthesis, characterization, and application in water treatment. Environmental Technology: 1–38.
Tran, H. N., Wang, Y.-F., You, S.-J., & Chao, H.-P. (2017c). Sustainable biochar derived from agricultural waste for removal of methylene Green 5 from aqueous solution: adsorption kinetic, isotherm, thermodynamic, and mechanism analysis.
Tran, H. N., You, S.-J., & Chao, H.-P. (2017d). Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. Journal of Environmental Management, 188, 322–336.
Wang, Z., Liu, G., Zheng, H., Li, F., Ngo, H. H., Guo, W., Liu, C., Chen, L., & Xing, B. (2015). Investigating the mechanisms of biochar’s removal of lead from solution. Bioresource Technology, 177, 308–317.
Xu, S., Liu, C., Ye, F., Guo, Y., & Wiezorek, J. (2017). Alkali-assisted hydrothermal route to control submicron-sized nanoporous carbon spheres with uniform distribution. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 515, 1–11.
Yang, Y., Shi, Q., Feng, J., Shu, X., & Feng, J. (2014). Preparation and antibacterial properties of an activated carbon sphere-quaternary phosphonium salt composite. RSC Advances, 4(92), 50708–50712.
Yu, L., Falco, C., Weber, J., White, R. J., Howe, J. Y., & Titirici, M.-M. (2012). Carbohydrate-derived hydrothermal carbons: a thorough characterization study. Langmuir, 28(33), 12373–12383.
Acknowledgements
This current work was financially supported by Chung Yuan Christian University (CYCU) in Taiwan. The first author would like to thank Chung Yuan Christian University for the Distinguished International Graduate Students (DIGS) scholarship to pursue his doctoral studies.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
ESM 1
(DOCX 2037 kb)
Rights and permissions
About this article
Cite this article
Tran, H.N., Lee, CK., Vu, M.T. et al. Removal of Copper, Lead, Methylene Green 5, and Acid Red 1 by Saccharide-Derived Spherical Biochar Prepared at Low Calcination Temperatures: Adsorption Kinetics, Isotherms, and Thermodynamics. Water Air Soil Pollut 228, 401 (2017). https://doi.org/10.1007/s11270-017-3582-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3582-3