Abstract
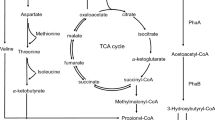
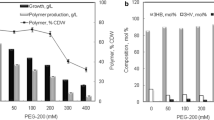
Bacillus megaterium strain OU303A isolated from municipal sewage sludge was selected for the study of biosynthesis of polyhydroxybutyrate (PHB) and polyhydroxybutyrate-co-hydroxyvalerate P (HB-co-HV) copolymer. The strain yielded a maximum of 62.43% DCW polymer in the medium containing glycerol as carbon source, which was followed by 58.63% DCW polymer in glucose containing medium. We found that this strain was capable of producing 2.5% hydroxyvalerate copolymer from a single carbon substrate, glucose. The strain showed an increase in the amount of HV monomer content, when the precursor for the copolymer was included in the fermentation medium. The characterization of the biopolymers was carried out using FTIR, GC-MS, H1 NMR and DSC. This is the first report of B. megaterium strain producing HV copolymer, without the addition of any precursor in the fermentation medium.





Similar content being viewed by others
References
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Anil Kumar PK, Shamala TR, Kshama L, Prakash MH, Joshi GJ, Chandrasekhar GJ, Lata Kumari KS, Divyashree MS (2007) Bacterial synthesis of poly(hydroxybutyrate-co-hydroxyvalerate) using carbohydrate-rich mahua (Madhuca sp) flowers. J Appl Microbiol 103:204–209. doi:10.1111/j.1365-2672.2006.03221.x
Bandrup J, Immergut EH (1989) Polymer handbook, 3rd edn. Interscience Press, New York
Borah B, Thakur PS, Nigam JN (2002) The influence of nutritional and environmental conditions on the accumulation of poly-β-hydroxybutyrate in Bacillus mycoides RLJ B-017. J Appl Microbiol 92:776–783. doi:10.1046/j.1365-2672.2002.01590.x
Caballero KP, Karel SF, Register RA (1995) Biosynthesis and characterization of hydroxybutyrate-hydroxycaproate copolymers. Int J Biol Macromol 17:86–92. doi:10.1016/0141-8130(95)93522-Y
Chen GQ, Konig KH, Lafferty RW (1991) Occurrence of poly-D (-)-3-hydroxyalkanoates in the genus Bacillus. FEMS Microbiol Lett 84:173–176
Dawes EA, Senior PJ (1973) The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol 10:135–266. doi:10.1016/S0065-2911(08)60088-0
Doi Y (1990) Microbial polyesters. VCH Publishers Inc, Yokohama, Japan
Doi Y, Kunioka M, Nakamura Y, Soga K (1986) Nuclear magnetic resonance studies on poly (β-hydroxybutyrate) and a popolymer of β-hydroxybutyrate and β-hydroxyvalerate isolated from Alcaligenes eutrophus H16. Macromol 19:2860–2864. doi:10.1021/ma00165a033
Eschenlauer AC, Stoup SK, Srienc F, Somers DA (1996) Production of heteropolymeric polyhydroxyalkanoate in Escherichia coli from a single carbon source. Int J Biol Macromol 19:121–130. doi:10.1016/0141-8130(96)01114-2
Labuzek S, Radecka I (2001) Biosynthesis of PHB terpolymer by Bacillus cereus UW85. J Appl Microbiol 90:353–357. doi:10.1046/j.1365-2672.2001.01253.x
Lagveen RG, Huisman GW, Preustig H, Ketelaar P, Eggnik G, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans; effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanotes and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54:2924–2932
Law KH, Leung YC, Lawford H, Chau H, Lo WH, Yu PH (2001) Production of polyhydroxybutyrate by Bacillus species isolated from municipal activated sludge. Appl Biochem Biotechnol 91–93:515–524. doi:10.1385/ABAB:91-93:1-9:515
Lee SY (1996) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14. doi:10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.0.CO;2-P
Madison LL, Huisman GW (1999) Metabolic engineering of poly (3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53
Quillaguaman J, Degado O, Mattiasson B, Kaul RH (2006) Poly (β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1. Enzyme Microb Technol 38:148–154. doi:10.1016/j.enzmictec.2005.05.013
Rawte T, Mavinkurve S (2002) A rapid hypochlorite method for the extraction of polyhydroxyalkanoates from bacterial cells. Indian J Exp Biol 40:924–929
Sasikala CH, Ramana CHV (1996) Biodegradable Polyesters. In: Neidleman SL, Laskin AI (eds) Advances in Applied Microbiology, vol 42. Academic Press, California, pp 97–218
Silverstein RM, Bassler GC, Morrill TC (1991) Spectrophotometric identification of organic compounds, 5th edn. Wiley, New York, pp 15–16
Sneath PHA (1986) Endospore-forming Gram-positive rods and cocci. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systemic bacteriology, vol 2. Williams and Wilkins, Baltimore, pp 1104–1139
Steinbüchel A (1991) Polyhydroxyalkanoic acids. In: Byrom (ed) Biomaterials: novel materials from biological sources. Stockton, New York, pp 124–213
Tajima K, Igari T, Nishimura D, Nakamura M, Satoh Y, Munekata M (2003) Isolation and characterization of Bacillus sp. INT005 accumulating polyhydroxyalkanoate (PHA) from gas field soil. J Biosci Bioeng 95:77–81
Valappil SP, Peiris D, Langley GJ, Herniman JM, Boccaccini AR, Bucke C, Roy I (2007) Polyhydroxyalkanoate (PHA) biosynthesis from structurally unrelated carbon sources by a newly characterized Bacillus spp. J Biotechnol 127:475–487. doi:10.1016/j.jbiotec.2006.07.015
Valappil SP, Rai R, Bucke C, Roy I (2008) Polyhydroxyalkanoate biosynthesis in Bacillus cereus SPV under varied limiting conditions and an insight into the biosynthetic genes involved. J Appl Microbiol 104(6):1624–1635. doi:10.1111/j.1365-2672.2007.03678.x
Acknowledgements
The authors thank UGC and DST for providing the facilities for this work through SAP and FIST programs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vishnuvardhan Reddy, S., Thirumala, M. & Mahmood, S.K. Production of PHB and P (3HB-co-3HV) biopolymers by Bacillus megaterium strain OU303A isolated from municipal sewage sludge. World J Microbiol Biotechnol 25, 391–397 (2009). https://doi.org/10.1007/s11274-008-9903-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9903-3