Abstract
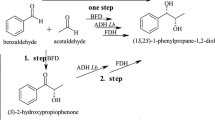
Enantiospecific microbial reduction of bicyclic ketones was described. Racemic Wieland–Miescher (1) and Hajos–Parrish (2) ketones were used as substrates. In a 4-h biotransformation of Hajos–Parrish ketone (2) using the strain of Didymosphaeria igniaria an optically pure ketone (R)-2 was obtained, whereas the (S)-2 ketone underwent reduction to (4aS,5S)-4 alcohol with 100% of enantiomeric excess and with over 60% of diastereoisomeric excess. Jones oxidation of the alcohol obtained in the biotransformation gave an optically pure ketone (S)-2. Enzymatic system of Coryneum betulinum reduced the (R)-2 ketone to (4aR,5S)-4 alcohol with a high enantiomerical purity in a 6-day reaction. Wieland-Miescher (1) ketone was transformed by these microorganisms in an analogous way, but the reaction times were longer.






Similar content being viewed by others
References
Andrade LH, Comasseto JV, Rodrigues DF, Pellizari VH, Porto ALM (2005) Enantioselective reduction of ortho-substituted-acetophenones by bacterial strains isolated from medium enriched with biphenyl or diesel fuel. J Mol Catal B Enzym 33:73–79
Bichlmaier I, Siiskonen A, Finel M, Yli-Kauhaluoma J (2006) Stereochemical sensitivity of the human UDP-glucuronosyltransferases 2B7 and 2B17. J Med Chem 49:1818–1827
Buchschachter P, Fürst A, Gutzwiller J (1990) (S)-8a-methyl-3, 4, 8, 8a-tetrahydro-1, 6(2H, 7H)-naphthalenedion. Org Synth Coll 7:368–372
Cheung WS, Wong HNC (1999) Total synthesis of (-)-hispanolone and an improved approach towards prehispanolone. Tetrahedron 55:11001–11016
Fuhshuku K, Funa N, Akeboshi T, Ohta H, Hosomi H, Ohba S, Sugai T (2000) Access to Wieland-Miescher ketone in an enantiomerically pure form by kinetic resolution with yeast-mediated reduction. J Org Chem 65:129–135
Fuhshuku K, Tomita M, Sugai T (2003) Enantiomerically pure octahydronaphthalenone and octahydroindenone: elaboration of the substrate overcame the specificity of yeast-mediated reduction. Adv Synth Catal 345:766–774
Grieco PA, Collins JL, Moher ED, Fleck TJ, Gross RS (1993) Synthetic studies on quassinoids: total synthesis of (-)-chaparrinone, (-)-glaucarubolone, and (+)-glaucarubinone. J Am Chem Soc 115:6078–6093
Hioki H, Hasimoto T, Kodama M (2000) Efficient kinetic resolution of (±)-4-methyl-Hajos-Parrish ketone by baker`s yeast reduction. Tetrahedron Asymmetr 11:829–834
Janeczko T, Dmochowska-Gładysz J, Białońska A, Ciunik Z (2006a) Microbial Hydroxylation of Chiral Bicyclic Enones by Chaetomium sp. 1 and Didymosphaeria igniaria Cultures. Biocat Biotrans 24:458–463
Janeczko T, Dmochowska-Gładysz J, Kuźbik M, Kowalski T (2006b) Enantiospecific microbial reduction of acetophenone and its methoxy derivatives. Chem Agric 7:181–185
Janeczko T, Dmochowska-Gładysz J, Kostrzewa-Susłow E (2009) Microbial enantioselective reduction of acetylpyridine. Przemysł Chemiczny 5:458–460
Kim M, Kawada K, Gross RS, Watt DS (1990) An enantioselective synthesis of (+)-picrasin B, (+)-Δ2-picrasin B, and (+)-Quassin from the R-(-)enantiomer of the Wieland-Miescher ketone. J Org Chem 55:504–511
Lin Y, Song X, Fu J, Lin J, Qu Y (2009) Microbial transformation of Androst-4-ene-3, 17-dione by Bordetella sp. B4 CGMCC 2229. J Chem Technol Biotechnol 84:789–793
Linder W, Rath M, Stoschitzky K, Semmelrock HJ (1989) Pharmacokinetic data of propranolol enantiomers in a comparative human study with (S)- and (R, S)-propranolol. Chirality 1:10–13
Shimizu N, Akita H, Kawamata T (2002) Enzymatic resolution of cis- and trans-1, 2, 3, 4, 6, 7, 8, 8a-octahydro-8a-methyl-6-oxo-naphtyl acetate derivatives. Tetrahedron Asymmetr 13:2123–2131
Yoe S-K, Hatae N, Seki M, Kanematsu K (1995) Enantioselective synthesis of an oxa-taxane derivative via tandem intramolecular [2 + 2] cycloaddition and [3, 3]-sigmatropic rearrangement of allenyl ether. Tetrahedron 51:3499–3506
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janeczko, T., Dmochowska-Gładysz, J. & Kostrzewa-Susłow, E. Chemoenzymatic resolution of racemic Wieland–Miescher and Hajos–Parrish ketones. World J Microbiol Biotechnol 26, 2047–2051 (2010). https://doi.org/10.1007/s11274-010-0390-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0390-y