Abstract
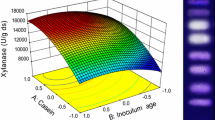
Humicola brevis var. thermoidea cultivated under solid state fermentation in wheat bran and water (1:2 w/v) was a good producer of β-glucosidase and xylanase. After optimization using response surface methodology the level of xylanase reached 5,791.2 ± 411.2 U g−1, while β-glucosidase production was increased about 2.6-fold, reaching 20.7 ± 1.5 U g−1. Cellulase levels were negligible. Biochemical characterization of H. brevis β-glucosidase and xylanase activities showed that they were stable in a wide pH range. Optimum pH for β-glucosidase and xylanase activities were 5.0 and 5.5, respectively, but the xylanase showed 80 % of maximal activity when assayed at pH 8.0. Both enzymes presented high thermal stability. The β-glucosidase maintained about 95 % of its activity after 26 h in water at 55 °C, with half-lives of 15.7 h at 60 °C and 5.1 h at 65 °C. The presence of xylose during heat treatment at 65 °C protected β-glucosidase against thermal inactivation. Xylanase maintained about 80 % of its activity after 200 h in water at 60 °C. Xylose stimulated β-glucosidase activity up to 1.7-fold, at 200 mmol L−1. The notable features of both xylanase and β-glucosidase suggest that H. brevis crude culture extract may be useful to compose efficient enzymatic cocktails for lignocellulosic materials treatment or paper pulp biobleaching.





Similar content being viewed by others
References
Adsul MG, Singhvi MS, Gaikaiwari SA, Gokhale DV (2011) Development of biocatalysts for production of commodity chemicals from lignocellulosic biomass. Bioresour Technol 102:4304–4312. doi:10.1016/j.biortech.2011.01.002
Bajpai P (2004) Biological bleaching of chemical pulps. Crit Rev Biotechnol 24:1–58. doi:10.1080/07388550490465817
Bhatia Y, Mishra S, Bisaria VS (2002) Microbial β-glucosidase: cloning, properties and applications. Crit Rev Biotechnol 22:375–407. doi:10.1080/07388550290789568
Bohlin C, Olsen SN, Morant MD, Patkar S, Borch K, Westh P (2010) A comparative study of activity and apparent inhibition of fungal β-glucosidases. Biotechnol Bioeng 107:943–952. doi:10.1002/bit.22885
Camassola M, Dillon AJP (2007) Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. J Appl Microbiol 103:2196–2204. doi:10.1111/j.1365-2672.2007.03458.x
Christopher L, Bissoon S, Singh S, Szendefy J, Szakacs G (2005) Bleach-enhancing abilities of Thermomyces lanuginosus xylanases produced by solid state fermentation. Process Biochem 40:3230–3235. doi:10.1016/j.procbio.2005.03.027
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23. doi:10.1016/j.femsre.2004.06.005
Da Silva R, Lago ES, Merheb CW, Macchione MM, Park YK, Gomes E (2005) Production of xylanase and CMCase on solid state fermentation in different residues by Thermoascus aurantiacus Miehe. Braz J Microbiol 36:235–241. doi:10.1590/S1517-83822005000300006
Damaso MCT, Andrade CMMC, Pereira P (2000) Use of corncob for endoxylanase production by thermophilic fungus Thermomyces lanuginosus IOC-4145. Appl Biochem Biotechnol 84–86:821–834. doi:10.1385/ABAB:84-86:1-9:821
Deswal D, Khasa YP, Kuhad RC (2011) Optimization of cellulase production by a brown rot fungus Fomitopsis sp. RCK2010 under solid state fermentation. Bioresour Technol 102:6065–6072. doi:10.1016/j.biortech.2011.03.032
Dhillon GS, Oberoi HS, Kaur S, Bansal S, Brar SK (2011) Value-addition of agricultural wastes for augmented cellulase and xylanase production through solid-state tray fermentation employing mixed-culture of fungi. Ind Crops Prod 34:1160–1167. doi:10.1016/j.indcrop.2011.04.001
Fang TJ, Liao BC, Lee SC (2010) Enhanced production of xylanase by Aspergillus carneus M34 in solid-state fermentation with agricultural waste using statistical approach. New Biotechnol 27:25–32. doi:10.1016/j.nbt.2009.09.008
Filho EXF (1996) Purification and characterization of a β-glucosidase from solid-state cultures of Humicola grisea var. thermoidea. Can J Microbiol 42:1–5. doi:10.1139/m96-001
Gaffney M, Doyle S, Murphy R (2009) Optimization of xylanase production by Thermomyces lanuginosus in solid state fermentation. Biosci Biotechnol Biochem 73:2640–2644. doi:10.1271/bbb.90493
Gao J, Weng H, Xi Y, Zhu D, Han S (2008) Purification and characterization of a novel endo-β-1,4-glucanase from the thermoacidophilic Aspergillus terreus. Biotechnol Lett 30:323–327. doi:10.1007/s10529-007-9536-x
Garg G, Mahajan R, Kaur A, Sharma J (2011) Xylanase production using agro-residue in solid-state fermentation from Bacillus pumilus ASH for biodelignification of wheat straw pulp. Biodegradation 22:1143–1154. doi:10.1007/s10532-011-9470-4
Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Zupancic S (1996) Production of fungal xylanases. Bioresour Technol 58:137–161. doi:10.1016/S0960-8524(96)00094-6
Harris D, Debolt S (2010) Synthesis, regulation and utilization of lignocellulosic biomass. Plant Biotechnol J 8:244–262. doi:10.1111/j.1467-7652.2009.00481.x
Hölker U, Höfer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186. doi:10.1007/s00253-003-1504-3
Jahromi MF, Liang JB, Rosfarizan M, Goh YM, Shokryazdan P, Ho YW (2011) Efficiency of rice straw lignocelluloses degradability by Aspergillus terreus ATCC 74135 in solid state fermentation. Afr J Biotechnol 10:4428–4435
Jatinder K, Chadha BS, Saini HS (2006a) Optimization of medium components for production of cellulases by Melanocarpus sp. MTCC 3922 under solid-state fermentation. World J Microbiol Biotechnol 22:15–22. doi:10.1007/s11274-005-2821-8
Jatinder K, Chadha BS, Saini HS (2006b) Regulation of cellulase production in two thermophilic fungi Melanocarpus sp. MTCC 3922 and Scytalidium thermophilum MTCC 4520. Enzyme Microb Technol 38:931–936. doi:10.1016/J.ENZMICTEC.2005.08.036
Jatinder K, Chadha BS, Saini HS (2006c) Optimization of culture conditions for production of cellulases and xylanases by Scytalidium thermophilum using response surface methodology. World J Microbiol Biotechnol 22:169–176. doi:10.1007/s11274-005-9015-2
Jiang Z, Cong Q, Yan Q, Kumar N, Dub X (2010) Characterisation of a thermostable xylanase from Chaetomium sp. and its application in chinese steamed bread. Food Chem 120:457–462. doi:10.1016/j.foodchem.2009.10.038
Joshi C, Khare SK (2011) Utilization of deoiled Jatropha curcas seed cake for production of xylanase from thermophilic Scytalidium thermophilum. Bioresour Technol 102:1722–1726. doi:10.1016/j.biortech.2010.08.070
Kalogeris E, Christakopoulos P, Kekos D, Macris BJ (1998) Studies on the solid-state production of thermostable endoxylanases from Thermoascus aurantiacus: characterization of two isozymes. J Biotechnol 60:155–163. doi:10.1016/S0168-1656(97)00186-7
Kamra P, Satyanarayana T (2004) Xylanase production by the thermophilic mold Humicola lanuginosa in solid-state fermentation. Appl Biochem Biotechnol 119:145–157. doi:10.1385/ABAB:119:2:145
Khucharoenphaisan K, Tokuyama S, Kitpreechavanich V (2010) Purification and characterization of a high-thermostable b-xylanase from newly isolated Thermomyces lanuginosusTHKU-49. Mycoscience 51:405–410. doi:10.1007/s10267-010-0054-7
Knob A, Terrasan CRF, Carmona EC (2010) β-Xylosidases from filamentous fungi: an overview. World J Microbiol Biotechnol 26:389–407. doi:10.1007/s11274-009-0190-4
Kumar R, Singh S, Singh OV (2008) Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol 35:377–391. doi:10.1007/s10295-008-0327-8
Leite RSR, Gomes E, da Silva R (2007) Characterization and comparison of termostability of purified β-glucosidases from a mesophilic Aureobasidium pullulans and a thermophilic Thermoacus aurantiacus. Process Biochem 42:1101–1106. doi:10.1016/j.procbio.2007.05.003
Leite RSR, Alves-Prado HF, Cabral H, Pagnocca FC, Gomes E, Da Silva R (2008) Production and characteristics comparison of crude β-glucosidases produced by microorganisms Thermoascus aurantiacus e Aureobasidium pullulans in agricultural wastes. Enzyme Microb Technol 43:391–395. doi:10.1016/j.enzmictec.2008.07.006
Lucena-Neto SA, Filho EXF (2004) Purification and characterization of a new xylanase from Humicola grisea var. thermoidea. Braz J Microbiol 35:86–90. doi:10.1590/S1517-83822004000100014
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. doi:10.1128/MMBR.66.3.506-577.2002
Maheshwari R, Bharadwaj G, Bhat MK (2000) Thermophilic fungi: their physiology and enzymes. Microbiol Mol Biol Rev 64:461–488. doi:10.1128/MMBR.64.3.461-488.2000
McIlvaine TC (1921) A buffer solution for colorimetric comparison. J Biol Chem 49:183–186
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Narang S, Sahai V, Bisaria VS (2001) Optimization of xylanase production by Melanocarpus albomyces IIS68 in solid state fermentation using response surface methodology. J Biosci Bioeng 91:425–427. doi:10.1016/S1389-1723(01)80164-X
Nascimento CV, Souza FHM, Masui DC, Leone FA, Peralta RM, Jorge JA, Furriel RPM (2010) Purification and biochemical properties of a glucose-stimulated β-d-glucosidase produced by Humicola grisea var. thermoidea grown on sugarcane bagasse. J Microbiol 48:53–62. doi:10.1007/s12275-009-0159-x
Peralta RM, Terenzi HF, Jorge JA (1990) β-D-Glycosidase activities of Humicola grisea: biochemical and kinetic characterization of a multifunctional enzyme. Biochim Biophys Acta 1033:243–249. doi:10.1016/0304-4165(90)90127-I
Peralta RM, Kadowaki MK, Terenzi HF, Jorge JA (1997) A highly thermostable β-glucosidase activity from the thermophilic fungus Humicola grisea var. thermoidea: purification and biochemical characterization. FEMS Microbiol Lett 146:291–295. doi:10.1111/j.1574-6968.1997.tb10207.x
Qing Q, Wyman CE (2011) Hydrolysis of different chain length xylooliogmers by cellulase and hemicellulase. Bioresour Technol 102:1359–1366. doi:10.1016/j.biortech.2010.09.001
Qing Q, Yang B, Wyman CE (2010) Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour Technol 101:9624–9630. doi:10.1016/j.biortech.2010.06.137
Rajoka MI, Akhtar MW, Hanif A, Khalid AL (2006) Production and characterization of a highly active cellobiase from Aspergillus niger grown in solid state fermentation. World J Microbiol Biotechnol 22:991–998. doi:10.1007/s11274-006-9146-0
Read SM, Northcote DH (1981) Minimization of variation in the response to different protein of the Coomassie blue G dye-binding assay for protein. Anal Biochem 116:53–64. doi:10.1016/0003-2697(81)90321-3
Riou C, Salmon JM, Vallier MJ, Gunata Z, Barre P (1998) Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl Environ Microbiol 64:3607–3614
Romdhane IBB, Maaleja-Chouri I, Belghith H (2010) Improvement of highly thermostable xylanases production by Talaromyces thermophilus for the agro-industrials residue hydrolysis. Appl Biochem Biotechnol 162:1635–1646. doi:10.1007/s12010-010-8945-9
Sharma M, Soni R, Nazir A, Oberoi HS, Chadha BS (2011) Evaluation of glycosyl hydrolases in the secretome of Aspergillus fumigatus and saccharification of alkali-treated rice straw. Appl Biochem Biotechnol 163:577–591. doi:10.1007/s12010-010-9064-3
Singh S, Madlala AM, Prior BA (2003) Thermomyces lanuginosus: properties of strains and their hemicellulases. FEMS Microbiol Rev 27:3–16. doi:10.1016/S0168-6445(03)00018-4
Singh S, Tyagi CH, Dutt D, Upadhyaya JS (2009) Production of high level of cellulase-poor xylanases by wild strains of white-rot fungus Coprinellus disseminatus in solid-state fermentation. New Biotechnol 26:165–170. doi:10.1016/j.nbt.2009.09.004
Singhania RR, Sukumaran RK, Patel AK, Larroche C, Pandey A (2010) Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb Technol 46:541–549. doi:10.1016/S0168-6445(03)00018-4
Soni R, Nazir A, Chadha BS, Saini HS (2008) Novel sources of fungal cellulases for efficient deinking of composite paper waste. Bioresources 3:234–246
Soni R, Nazir A, Chadha BS (2010a) Optimization of cellulase production by a versatile Aspergillus fumigatus fresenius strain (AMA) capable of efficient deinking and enzymatic hydrolysis of Solka floc and bagasse. Ind Crops Prod 31:277–283. doi:10.1016/j.indcrop.2009.11.007
Soni SK, Batra N, Bansal N, Soni R (2010b) Bioconversion of sugarcane bagasse into second generation bioethanol after enzymatic hydrolysis with in-house produced cellulases from Aspergillus sp. S4B2F. Bioresources 5:741–758
Sonia KG, Chadha BS, Badhan AK, Saini HS, Bhat MK (2008) Identification of glucose tolerant acid active β-glucosidases from thermophilic and thermotolerant fungi. World J Microbiol Biotechnol 24:599–604. doi:10.1007/s11274-007-9512-6
Souza FHM, Nascimento CV, Rosa JC, Masui DC, Leone FA, Jorge JA, Furriel RPM (2010) Purification and biochemical characterization of a mycelial glucose- and xylose-stimulated β-glucosidase from the thermophilic fungus Humicola insolens. Process Biochem 45:272–278. doi:10.1016/j.procbio.2009.09.018
Takó M, Tóth A, Nagy LG, Krisch J, Vágvolgyi C, Papp T (2010) A new β-glucosidase gene from the zygomycete fungus Rhizomucor miehei. Antonie Van Leeuwenhoek 97:1–10. doi:10.1007/s10482-009-9382-z
Techapun C, Poosaran N, Watanabe M, Sasaki K (2003) Thermostable and alkaline-tolerant microbial cellulase-free xylanases produced from agricultural wastes and the properties required for use in pulp bleaching bioprocesses: a review. Process Biochem 38:1327–1340. doi:10.1016/S0032-9592(02)00331-X
Uchima CA, Tokuda G, Watanabe H, Kitamoto K, Arioka M (2011) Heterologous expression and characterization of a glucose-stimulated β-glucosidase from the termite Neotermes koshunensis in Aspergillus oryzae. Appl Microbiol Biotechnol 89:1761–1771. doi:10.1007/s00253-010-2963-y
Vafiadi C, Christakopoulos P, Topakas E (2010) Purification, characterization and mass spectrometric identification of two thermophilic xylanases from Sporotrichum thermophile. Process Biochem 45:419–424. doi:10.1016/j.procbio.2009.10.009
Xu H, Xiong AS, Zhao W, Tian YS, Peng RH, Chen JM, Yao QH (2011) Characterization of a glucose-, xylose-, sucrose-, and d-galactose-stimulated β-glucosidase from the alkalophilic bacterium Bacillus halodurans C-125. Curr Microbiol 62:833–839. doi:10.1007/s00284-010-9766-3
Yang S, Jiang Z, Yan Q, Zhu H (2008) Characterization of a thermostable extracellular β-glucosidase with activities of exoglucanase and transglycosylation from Paecilomyces thermophila. J Agric Food Chem 56:602–608. doi:10.1021/jf072279+
Zanoelo FF, Polizeli MLTM, Terenzi HF, Jorge JA (2004) β-Glucosidase activity from the thermophilic fungus Scytalidium thermophilum is stimulated by glucose and xylose. FEMS Microbiol Lett 240:137–143. doi:10.1016/j.femsle.2004.09.021
Acknowledgments
This investigation was supported by research grants from the Conselho de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). DCM received a post-doctoral scholarship from CNPq. FHMS received a PhD. scholarship from FAPESP, and ALRLZ a doctoral scholarship from CAPES and FAPESP. LHSG, RPMF and JAJ received a research scholarship from CNPq. We thank Nilton Rosa Alves and Mauricio de Oliveira for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masui, D.C., Zimbardi, A.L.R.L., Souza, F.H.M. et al. Production of a xylose-stimulated β-glucosidase and a cellulase-free thermostable xylanase by the thermophilic fungus Humicola brevis var. thermoidea under solid state fermentation. World J Microbiol Biotechnol 28, 2689–2701 (2012). https://doi.org/10.1007/s11274-012-1079-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1079-1