Abstract
Pannexin 1 (Panx1) is a ubiquitously expressed protein forming large conductance channels that are central to many distinct inflammation and injury responses. There is accumulating evidence showing ATP released from Panx1 channels, as well as metabolites, provide effective paracrine and autocrine signaling molecules that regulate different elements of the injury response. As channels with a broad range of permselectivity, Panx1 channels mediate the secretion and uptake of multiple solutes, ranging from calcium to bacterial derived molecules. In this review, we describe how Panx1 functions in response to different pro-inflammatory stimuli, focusing mainly on signaling coordinated by the vasculature. How Panx1 mediates ATP release by injured cells is also discussed. The ability of Panx1 to serve as a central component of many diverse physiologic responses has proven to be critically dependent on the context of expression, post-translational modification, interacting partners, and the mode of stimulation.
Similar content being viewed by others
Introduction
Stimulated secretion of cytosolic metabolites, including ATP, is an important mechanism for paracrine and autocrine signaling. Among several mechanisms of ATP release, including plasma membrane channels, vesicular exocytosis, and extracellular synthesis [1], pannexin channels have emerged as an important mediator of ATP secretion that regulates multiple processes involved in inflammation and other injury responses.
Pannexins were discovered in 2000 [2], based on a search for mammalian proteins that are homologous to invertebrate gap junction proteins, innexins [3]. Initially, it was thought that pannexins were functionally equivalent to the classical mammalian gap junction proteins, connexins. However, instead of assembling into gap junctions [4], the vast preponderance of evidence supports a role for pannexins as plasma membrane channels (and not hemichannels), forming a high conductance pore that can mediate the release of cytosolic ions and metabolites, including ATP.
Pannexin channels have been linked to pathophysiological processes related to the vasculature, including hypertension [5,6,7,8], stroke [9, 10], and inflammation/ischemic injury [11,12,13,14,15], possibly including complications due to COVID-19 [16]. In this review, we summarize current progress in identifying roles for the most ubiquitous pannexin isoform, Panx1, in regulating how the vasculature reacts to injury and inflammation.
Pannexin 1 structure, function, and post-translational modifications
There are three pannexin isoforms (Panx1, Panx2, Panx3) that exhibit a high degree of amino acid sequence conservation [17,18,19]. It is not common to observe more than one pannexin isoform in a cell type, although Panx1 is fairly ubiquitous. Panx2 is expressed in the central nervous system, and Panx3 can be found in the skin, osteoblasts, or specialized cartilage of the mouse [18, 20]. Elements of the vasculature show differential pannexin expression as well [21]. Although their expression is highly regulated, pannexins share considerable functional redundancy. This is underscored by several studies using pannexin knockout mice demonstrating that global knockout of one pannexin is compensated by upregulation of another isoform [22,23,24,25].
Pannexins form large conductance, relatively non-selective channels that can mediate permeability of both anionic and cationic molecules up to 1 kDa in size [26]. Molecules passively diffuse through Panx1 channels, following concentration gradients, and as solute size increases, the permselectivity of Panx1 favors anions over cations [27]. Pannexin 1 forms gated channels, with multiple subconductance states which can also regulate solute permselectivity [28]. Because of the relatively broad selectivity of Panx1 and the confounding effects of other channels in situ, it has proven challenging to directly attribute permeability of specific substrates to Panx1; however, recently, proteoliposomes containing purified Panx1 have been used to directly demonstrate and measure channel permeability and ATP release [26].
The vast majority of work has focused on Panx1 as an ATP channel, with multiple stimuli inducing ATP secretion as a paracrine signaling pathway. However, physiologic roles for Panx1 are not limited to secretion, as more recent evidence has shown that Panx1 can also act as a plasma membrane calcium channel, increasing cytosolic calcium in response to stimuli, such as TNFα [29, 30]. Several other solutes have been shown to be transmitted through Panx1 including fluorescent tracers, anandamide, lactate, glutamate, spermidine, and bacterial products, consistent with roles for Panx1 in multiple different signaling pathways [26, 27, 31,32,33].
Endogenous pannexins typically oligomerize into homomeric channels, although it is possible to generate heteromeric channels in vitro using transfected cells expressing two or three different pannexins [34]. Initially, it was thought that Panx1 forms hexamers [17], comparable to connexin hemichannels [35]; however, several labs, remarkably all at once in 2020, provided cryo-EM evidence that Panx1 forms heptameric channels [36,37,38,39,40,41]. There is evidence suggesting that Panx2 may form octomers [17] and the oligomerization state of Panx3 has not been determined. In general, little is known about how pannexin oligomerization is regulated and this is an area in need of additional research that may provide more insight into structure/function relationships of Panx1, as well as the other pannexin channels.
Evidence suggests Panx1 preferentially occupies caveolin-based cholesterol-enriched membrane microdomains (lipid rafts) [42]. In terms of other purinergic signaling components, this is a logical location for Panx1 channels since localization to rafts has the potential to facilitate complex formation with other components involved in Panx1 signaling that are also localized to caveolin-based lipid domains, e.g., CD73 and CD39 [43, 44], creating a localized signaling microdomain for purinergic-based signaling.
Panx1 is an exceptionally long lived protein, in contrast with connexins, that have a rapid rate of turnover (reviewed in [45, 46]). Although Panx1 turnover has not been measured using traditional pulse-chase assays, transient stimulation of endothelial cells with TNFα causes a spike in Panx1 protein content that takes 6–7 days to return to baseline levels [29]. In addition, the presence of Panx1 in red blood cells, which lack mRNA, also suggests that Panx1 can remain active for an extended amount of time in the plasma membrane [47]. Delivery to the plasma membrane is an exquisitely regulated process for Panx1, mediated by motifs and/or post-translational modifications (see below) [48, 49]. Once at the plasma membrane, strong evidence has demonstrated that extracellular ATP itself can induce internalization of the channel, providing an important negative feedback loop for Panx1 channel regulation [50, 51].
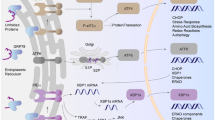
There are several post-translational modifications of Panx1 that can regulate trafficking and function including glycosylation, S-nitrosylation, caspase cleavage, and Src activation (Fig. 1). N-glycosylation is a critical post-translational modification facilitating trafficking, quality control, and protein folding of Panx1. N-glycosylation consensus sites are present in all three pannexins and have been shown to control the intracellular localization of pannexins and how they interact with other members of the pannexin family [34]. The importance of N-glycosylation for Panx1 processing and function is underscored by recent discoveries that mutations affecting Panx1 glycosylation are associated with cancer, infertility, and neonatal lethality [52, 53].
Caspase cleavage of Panx1 was first described as a mechanism to permanently activate channel opening [54] and increase channel permeability [26]. Caspase 3 and 7 cleaves the human Panx1 isoform [55] at residues 376–379, resulting in a constitutively open and active channel. Because of this constitutive opening, the cells are apoptosing, and cell death is imminent. In this state, ATP is released as a “find-me” signal from apoptotic immune cells in order to recruit macrophages, which is critical for a proper immune response [54].
S-nitrosylation is an important post-translational modification observed extensively in the vasculature and acts by adding a nitrosyl group to cysteine (C) residues to reversibly alter protein function [56]. Lohman et al. showed that nitrosylation of either C40 (N-terminus) or C346 (carboxyl tail) residues [57] potently inhibited Panx1 function, with other cysteines (e.g., C426) having no observable change. They showed the ATP and channel current inhibited by NO donors GSNO and DEA NONOate. This post-translational modification may be especially an important regulator in the nitrosothiol-rich circulation.
Multiple lines of evidence demonstrate an important role for sarcoma (Src) family kinases (SFKs) in Panx1 channel regulation [58] at tyrosine (Y) sites Y150 [52], Y198 [6], and Y308 [10]. Dynamic regulation of the Y150 site was shown to be required for complex N-glycosylation and overall trafficking of Panx1 to the plasma membrane. This is in comparison to constitutive phosphorylation of Y198 on the intracellular loop of Panx1 which was found to be important for Panx1 channel opening. A mimetic peptide against this region (“PxIL2P”) was able to inhibit Panx1 current, ATP release, and alpha-adrenergic vasoconstriction; more general Panx1 inhibition was also noted in endothelium and renin-secreting cells [5,6,7, 30, 49, 59]. Although the Y308 site has not been found to be as important in terms of plasma membrane localization or trafficking, it is similar to Y198, in that it was important for gating properties, including ATP release and current [10, 60]. Peptides against this region (“TAT-Panx308”) were also found to be potent inhibitors and were associated with decreased plaque size after stroke [60].
With respect to peptide inhibition of Panx1, 10Panx1 was the first “specific” pannexin blocker that had demonstrated some specificity in an in vitro based system [61]. Unlike the physiological effects observed with PxIL2P or TAT-Panx308, inhibition of Panx1 channel conductance by 10Panx1 in vivo has not been demonstrated. Interestingly, the connexin hemichannel peptide mimetics have also been shown to block Panx1 channels, indicating these inhibitors may be helpful in blocking all large-pore channels rather than just connexin hemichannels alone [62]. Pharmacologically, several inhibitors that are currently utilized show varying degrees of specificity towards pannexins, including mefloquine [63], probenecid [8, 64], trovafloxacin [65], and carbenoxolone [66]. Surprisingly, one of the most potent and specific inhibitors in terms of blocking Panx1 specifically, and not other large-pore channels (e.g., Cx43 or Panx2), is the anti-hypertensive spironolactone [7]. Both spironolactone and trovafloxacin were identified using unbiased screens for Panx1 inhibitors and both blocked Panx1 as demonstrated by single-channel recordings and inhibition of ATP release.
It is now clear genetic deletion of pannexins should preferentially utilize cell type-specific, inducible animals whenever possible. Evidence from multiple laboratories has demonstrated that other pannexins (e.g., Panx3) are upregulated to compensate for loss of Panx1 in global knockout mice (e.g., [22,23,24,25]). This is not necessarily an usual finding, considering that global knockouts must compensate during development for the loss of essential proteins (e.g., similar findings are observed with connexins [67]). In addition, sex dimorphism is another potential explanation for apparently discordant results observed for global Panx1 knockout mice [68].
Inflammation and sepsis
ATP release from Panx1 channels has been implicated in several aspects of inflammation. For instance, ATP release via Panx1 channels is necessary for appropriate clearance of apoptotic cells [54], where it acts as a “find-me” signal to instruct the chemotaxis of local immune cells to locate apoptotic cells. ATP release is also stimulated by TNFα, a key mediator of inflammation, where it promotes immune cell transmigration [11]. Panx1 has also been shown to amplify the effects of chronic TNFα exposure on endothelial cells by promoting the secretion of IL-1β through a Ca+2 and NFκB-dependent pathway [59]. This result highlights a dual role for Panx1 in regulating the acute phase of inflammation (via ATP release) and chronic phase (via Ca+2 uptake) (Fig. 2). This differential role of Panx1 is most likely due to differences in conformation or post-translational modifications leading to different subconductance states. Further work is needed to determine mechanisms that underlie the differential regulation of Panx1 during acute versus chronic inflammation.
In addition to releasing ATP, TNFα stimulated ATP secreted by venous endothelial cells can be metabolized by the CD39/CD73 complex to generate adenosine, which stimulates TRPV4 channels to increase vessel permeability [12]. The Panx1 inhibitor spironolactone was shown to be an effective inhibitor of vascular leak [12]. This suggests a potential Panx-specific pharmacological approach to treat edema associated with inflammation (e.g., pulmonary edema due to sepsis demonstrated using the cecal ligation and puncture model), since venous endothelial cells can distinguish ATP as a homing signal from adenosine as a permeability signal. This enables venules to coordinate an orderly transmigration response to inflammation. In this model, ATP release is an early event that attracts cells to sites of damage. Once recruited, hydrolysis of ATP to adenosine increases vessel permeability, making them more amenable to transmigration of inflammatory cells. Note also that ATP hydrolysis releases immune cells from strong attachment to the endothelium which also stimulates migration across the vascular barrier [69, 70].
In addition to preventing pulmonary edema in response to sepsis, Panx1 deficient mice and mice treated with spironolactone had an increased lifespan following cecal ligation and puncture [12]. This is consistent with reports showing that targeting Panx1 can have a beneficial effect in endotoxemia and models of sepsis [71,72,73]. Considering that sepsis is a significant public health concern, the ability to target Panx1 to control the severity of sepsis is an appealing pharmacologic approach.
Ischemia–reperfusion injury
The interruption of blood flow (ischemia) is catastrophic in that it prevents tissue oxygenation leading to an imbalance between metabolic demand and supply, resulting in tissue hypoxia [74]. Ischemia can be due to multiple insults including thrombosis, atherosclerotic plaques, embolism, and surgically clamped vessels. However, the subsequent restoration of blood flow (reperfusion) and resultant reoxygenation creates a significant injury response called ischemia–reperfusion injury (IRI) [14]. IRI is often accompanied by significant tissue damage and a severe inflammatory response [74, 75]. It also involves a complex cascade of processes involving the interplay between innate immune cells and endothelial cells [76].
IRI is implicated in morbidity and mortality in a broad spectrum of pathologies, such as cardiac arrest, acute kidney injury, myocardial infarction, ischemic stroke, trauma, sickle cell disease, and sleep apnea. IRI is also an important comorbidity associated with organ transplant, cardiac, and vascular surgeries [74]. In transplant, IRI has been linked to higher morbidity and mortality by increasing chances of graft rejection and may lead to graft damage [77]. Interestingly, multiorgan failure after inflammatory activation may be a consequence of IRI in a single organ, consistent with a systemic response to local disruption of blood flow [78]. Increased microvascular permeability is another consequence of IRI that is especially dangerous in lung transplant patients as it may lead to respiratory failure due to dysfunction of the alveolar-capillary barrier [15, 79].
During ischemia, anaerobic metabolism in cells predominates, which lowers ATP production and cellular pH. This is due in part to mitochondrial damage, where subsequent impairment of ATP production is caused by the dissipation of membrane potential after the opening of the mitochondrial permeability transition pore (MPT) [76]. Consequently, the Na+/H+ exchanger releases excess hydrogen ions from the cytosol, causing an influx in sodium [80, 81]. In addition, there is an increase in intracellular calcium in response to ischemia because decreased ATP levels are unable to support efficient Ca2+ efflux and sequestration. Reperfusion restores oxygen supply, and aerobic ATP production is again possible, which restores normal pH. However, there are several deleterious consequences of reperfusion, including endothelial dysfunction, inflammatory responses, and generation of reactive oxygen species (ROS) [75].
Purinergic signaling has a pivotal role during IRI, since ATP is released from apoptotic cells, activated endothelium, inflammatory cells, and necrotic cells [33]. ATP accumulation acts as a “find me” signal, which recruits phagocytes to sites of injury promoting the chemotaxis of inflammatory cells or activating the Nlrp3 inflammasome [74]. This promotes the release of the key cytokines that primarily regulate the vascular cell phenotype during acute systemic inflammation, including IL-1β and TNFα, intensifying IRI by creating an over-exuberant inflammatory response [82]. Because IRI is so closely related to circulating levels of ATP, the role for pannexins has been an active area of research.
Brain
Panx1 expression has been found through the brain, including in neurons [83,84,85], astrocytes [86], microglia [87], and cerebral endothelial and smooth muscle cells [9, 88]. Early studies demonstrated the opening of a large conductance plasma membrane channel in hippocampal pyramidal neurons due to oxygen–glucose deprivation (OGD) [89]. This channel was found to be Panx1, and it was postulated that opening of Panx1 channels by OGD was responsible for increased membrane permeability seen in necrotic cell death of neurons and that release of molecules such as ATP and glucose through Panx1 channels might compromise neuronal recovery [89].
Later work established that Panx1 channels are physiologically opened by N-methyl-d-aspartate receptor (NMDAR) stimulation [90]. This finding drove work to define roles of the NMDAR-Panx1 pathway under ischemic conditions, since NMDARs drive ischemic neuronal death through excitotoxicity that dysregulates intracellular Ca2+ homeostasis [91]. It should be noted that it was initially reported that Panx1 opening in OGD was NMDAR-independent [89] and that Panx1 was activated by other components of ischemia, including increased extracellular K+ [92].
Anoxic neurons can aberrantly depolarize, which was observed in hippocampal pyramidal neurons driven by NMDAR activation and subsequent opening of Panx1 channels. Panx1 opening in this context was mediated through activation of sarcoma (Src) family kinase leading to phosphorylation at Y308 on the C-terminal domain of Panx1 [10]. Further work delineated that although NMDAR activation was necessary for ischemia-induced excitotoxicity, neuronal blebbing and cell death occurred as a result of Panx1 channel activity and was not dependent on NMDAR pore function [60]. Blockade of the NMDAR pore with MK-801 or physiological Mg2+ did not block excitotoxic blebbing or Panx1 secondary currents in hippocampal pyramidal neurons. Instead, this suggested that NMDAR signaling drives Src kinase-mediated opening of Panx1 channels, resulting in neuronal excitotoxicity [60]. Critically, blockade of this Panx1 using TAT-Panx308 reduced infarct volumes and improved sensorimotor deficits after Middle Cerebral Artery Occlusion (MCAO) in rats [60], a finding that has been replicated with probenecid in mice [93].
ATP release through Panx1 channels might also activate ionotropic, Ca2+ permeable P2X7 receptors, leading to further Ca2+ influx and activation of apoptosis. This P2X7-Panx1 complex has been implicated in ischemic cell death, spreading cortical depression and associated neuroinflammation [94,95,96]. Specifically, ATP release from Panx1, resulting in autocrine P2X7R stimulation, is important for inflammasome assembly in neurons and astrocytes, and ultimately release of mature IL1β [94], especially in combination with endothelial stimulation with TNFα [59].
Recent studies have also found the importance of Panx1 in cerebral stroke outside of neurons. Genetic deletion of Panx1 from endothelial cells, but not smooth muscles, reduced ischemic stroke infarct size [9]. In these experiments, both arterial vasoconstriction in response to pressure changes, and infiltration of circulating leukocytes, were reduced in mice lacking endothelial Panx1 suggesting a dual role for endothelial Panx1 in the cerebral vasculature [9]. In addition to endothelial Panx1-dependent regulation of inflammation, Panx1 expression in myeloid cells, both microglia and circulating myeloid cells, was found to be important following a traumatic brain injury, which induces an ischemic injury secondary to the primary impact injury. Genetic deletion of Panx1 in myeloid cells using the Cx3Cr1 driven Cre recombinase reduced the presence of pro-inflammatory macrophages and neutrophils and improved functional motor skills [97]. Additional studies will need to be conducted to explore microglia cell activation versus infiltrated activated macrophages; however, together, these studies strongly suggest an important role for Panx1, both in endothelial and myeloid cells, in regulating neuroinflammation following a cerebral ischemic injury.
Kidney
In addition to the brain, the kidney is highly vulnerable to hypoxic injury [98]. ATP release is strongly associated with acute kidney injury (AKI) due to cell damage that takes place during disease progression, which is accompanied by worsening inflammation [99]. Panx1 is present throughout the kidney, especially in the vasculature, apical membranes of proximal tubules, collecting ducts, and thick descending limbs [100]. Regulation of ATP levels by Panx1 mediated release in the kidney is essential under physiological conditions, since it regulates renal hemodynamics and tubular transport in addition to pathological conditions [101]. Pharmacological and genetic screening methods of assessing the effects of Panx1 on renal IRI [14], a mouse model of AKI, revealed that global, endothelial, and epithelial tissue-specific deletion of Panx1 had a protective effect, as did pharmacological inhibition of Panx1 channels during AKI [14, 102]. Clearly, Panx1 is having a major physiological role in the physiology and pathology of the kidney; however, there has not been extensive investigation.
Heart
Panx1 has also been shown to play a role in IRI in the heart. Multiple studies have now demonstrated an increase in Panx1 expression following coronary IRI [103, 104]. In addition, murine atrial myocytes in vitro exposed to hypoxia induced ATP release in a Panx1-dependent manner [104]. It was hypothesized that the cardiomyocyte-dependent increase in extracellular ATP could activate surrounding fibroblasts and initiate the transformation into a myofibroblast phenotype [104]. Additionally, Panx1 has been demonstrated to play a role in pre-conditioning, short periods of ischemia followed by reperfusion that can protect the heart against longer periods of ischemic injuries [105]. Indeed, both Panx1 and P2X7 channel inhibitors prevented the pre-conditioning protective effect and resulted in larger infarcts following pre-conditioning and a subsequent IRI [105]. The release of cardioprotectants during pre-conditioning, which may include ATP, appears to be Panx1-P2X7-channel dependent [105]; however, it remains unknown which cell types expressing Panx1 are important for this protective role during pre-conditioning of the heart.
It is possible that expression of Panx1 in the coronary vasculature could have important roles in regulating vascular tone as well as inflammation. Previous studies have found that P2Y2 receptor-mediated vasoconstriction is reduced in coronary arteries following IRI, possibly due to a compensatory mechanism to combat the increased extracellular ATP during IRI [103]. Deletion of endothelial Panx1 or pharmacological inhibition of Panx1 at time of reperfusion protected cardiac function following IRI, although without a change in infarct volume [13]. This is hypothesized to be due to a reduction in pro-inflammatory macrophage infiltration into the non-injured cardiac tissue [13]. Together, these data suggest an important role for Panx1 in myocardial infarction; however, further studies are needed to understand the cell type-specific roles and potential beneficial versus detrimental roles of Panx1 in determining post-IRI cardiac function and outcome.
Lung
Transplanted lungs are particularly susceptible to IRI. Using a hilar ligation model revealed roles for Panx1 in the IRI response, which includes pulmonary edema, inflammation, neutrophil infiltration, increased microvascular permeability, and lung dysfunction (increased airway resistance, decreased compliance, and increased pulmonary artery pressure) [15]. All of these pathological consequences of lung IRI were ameliorated with the Panx1 inhibitors carbenoxolone or probenecid. Endothelial specific Panx1 deficient mice also were resistant to the effects of IRI on the lung, suggesting that Panx1 is a viable target to prevent the deleterious effects of IRI in lung transplant. Whether this is the case and whether it will translate to a protective effect in other transplant models remains to be determined.
Other organ systems
Although the above is generally focused on inflammation involved with IRI, there are other organ systems where pannexin-mediated inflammation may also play an important role in disease etiology, including: atherosclerosis [106], colitis [107], and multiple sclerosis [108]. The role of Panx1 continues to evolve as an important inflammatory mediator.
Conclusions and future directions
Given that Panx1 channels are relatively non-selective, it is remarkable that Panx1 is central to the regulation of many diverse processes. In many ways, this may be because Panx1 is flexible in its ability to interact with multiple different receptors. Panx1 also is heavily influenced by multiple different classes of post-translational modifications, meaning it can be regulated by multiple signal transduction pathways. As such, understanding the context of Panx1 expression and stimulation are critical to determining how it can regulate inflammation and injury responses, especially when considering Panx1 as a pharmacological target to prevent the debilitating pathology of diseases such as sepsis and tissue damage due to IRI.
Spironolactone has been shown to be a highly effective Panx1 inhibitor that does not affect other pannexins and connexin hemichannels, which makes it an important tool to define roles for Panx1 signaling and it may have therapeutic application [7, 12]. However, like all drugs, spironolactone does have multiple targets (e.g., mineralocorticoid receptor antagonist) and so there is a need for further drug discovery [7]. The ability to target Panx1 by repurposing other drugs, such as probenecid, identifying novel small molecules, and developing peptide-based drugs, such as PxIL2P, is anticipated to expand the scope of Panx1 as an effective pharmacologic target.
Most research on Panx1 has focused on its role in ATP secretion. However, it is clear that a broad range of molecules can be transmitted through Panx1, as a channel mediating both secretion and uptake (e.g., [26, 33]). The detailed structural models that have recently been developed will help identify key residues that control channel permselectivity that can be validated by expressing mutants in transfected cells or reconstituted in liposomes.
While global Panx1 knockout mice were critical to identifying initial roles for Panx1 in many different physiologic processes, they suffer from significant pitfalls due to compensatory expression of other proteins. Tissue-specific, inducible transgenic Panx1 deficient mice have provided a more specific way to target Panx1, which is especially critical for distinguishing roles for Panx1 in the endothelium versus leukocytes, both of which are involved in inflammation. Future research including mice expressing Panx1 point mutants targeting key regulatory sites will provide a deeper understanding of the cellular mechanisms that regulate Panx1 and enable it to be central to so many diverse physiological and pathophysiologic processes.
Data availability
No experimental data was generated for this review.
References
Lohman AW, Billaud M, Isakson BE (2012) Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res 95:269–280
Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S (2000) A ubiquitous family of putative gap junction molecules. Curr Biol 10:R473–R474
Phelan P, Bacon JP, Davies JA, Stebbings LA, Todman MG, Avery L, Baines RA, Barnes TM, Ford C, Hekimi S, Lee R, Shaw JE, Starich TA, Curtin KD, Sun YA, Wyman RJ (1998) Innexins: a family of invertebrate gap-junction proteins. Trends Genet 14:348–349
Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G (2011) Pannexin channels are not gap junction hemichannels. Channels (Austin) 5:193–197
Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE (2011) Pannexin1 regulates alpha1-adrenergic receptor-mediated vasoconstriction. Circ Res 109:80–85
Billaud M, Chiu YH, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, Somlyo AV, Thompson RJ, Le TH, Ravichandran KS, Bayliss DA, Isakson BE (2015) A molecular signature in the pannexin1 intracellular loop confers channel activation by the alpha1 adrenoreceptor in smooth muscle cells. Sci Signal 8:ra17
Good ME, Chiu YH, Poon IKH, Medina CB, Butcher JT, Mendu SK, DeLalio LJ, Lohman AW, Leitinger N, Barrett E, Lorenz UM, Desai BN, Jaffe IZ, Bayliss DA, Isakson BE, Ravichandran KS (2018) Pannexin 1 channels as an unexpected new target of the anti-hypertensive drug spironolactone. Circ Res 122:606–615
Nyberg M, Piil P, Kiehn OT, Maagaard C, Jorgensen TS, Egelund J, Isakson BE, Nielsen MS, Gliemann L, Hellsten Y (2018) Probenecid inhibits alpha-adrenergic receptor-mediated vasoconstriction in the human leg vasculature. Hypertension 71:151–159
Good ME, Eucker SA, Li J, Bacon HM, Lang SM, Butcher JT, Johnson TJ, Gaykema RP, Patel MK, Zuo Z, Isakson BE (2018) Endothelial cell Pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight 3:e96272
Weilinger NL, Tang PL, Thompson RJ (2012) Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci 32:12579–12588
Lohman AW, Leskov IL, Butcher JT, Johnstone SR, Stokes TA, Begandt D, DeLalio LJ, Best AK, Penuela S, Leitinger N, Ravichandran KS, Stokes KY, Isakson BE (2015) Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat Commun 6:7965
Maier-Begandt D, Comstra HS, Molina SA, Kruger N, Ruddiman CA, Chen YL, Chen X, Biwer LA, Johnstone SR, Lohman AW, Good ME, DeLalio LJ, Hong K, Bacon HM, Yan Z, Sonkusare SK, Koval M and Isakson BE (2021) A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFalpha-induced increases in endothelial permeability. Sci Signal 14:eaba2940
Good ME, Young A, Wolpe AG, Ma M, Johnstone SR, Hall PJ, Duffy CK, Aronovitz M, Martin G, Blanton RM, Leitinger N, Wolf MJ, Isakson BE (2021) Endothelial Pannexin 1 regulates cardiac response to myocardial infarction. Circ Res 128:1211–1213
Jankowski J, Perry HM, Medina CB, Huang L, Yao J, Bajwa A, Lorenz UM, Rosin DL, Ravichandran KS, Isakson BE, Okusa MD (2018) Epithelial and endothelial Pannexin1 channels mediate AKI. J Am Soc Nephrol 29:1887–1899
Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME, Lorenz UM, Kron IL, Bayliss DA, Ravichandran KS, Isakson BE, Laubach VE (2018) Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 315:L301–L312
Swayne LA, Johnstone SR, Ng CS, Sanchez-Arias JC, Good ME, Penuela S, Lohman AW, Wolpe AG, Laubach VE, Koval M, Isakson BE (2020) Consideration of Pannexin 1 channels in COVID-19 pathology and treatment. Am J Physiol Lung Cell Mol Physiol 319:L121–L125
Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE (2010) Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem 285:24420–24431
Penuela S, Bhalla R, Gong X-Q, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW (2007) Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci 120:3772–3783
Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y (2004) The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83:706–716
Le Vasseur M, Lelowski J, Bechberger JF, Sin WC, Naus CC (2014) Pannexin 2 protein expression is not restricted to the CNS. Front Cell Neurosci 8:392
Lohman AW, Billaud M, Straub AC, Johnstone SR, Best AK, Lee M, Barr K, Penuela S, Laird DW, Isakson BE (2012) Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res 49:405–416
Penuela S, Kelly JJ, Churko JM, Barr KJ, Berger AC, Laird DW (2014) Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J Invest Dermatol 134:2026–2035
Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M (2011) Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A 108:20772–20777
Whyte-Fagundes P, Kurtenbach S, Zoidl C, Shestopalov VI, Carlen PL, Zoidl G (2018) A potential compensatory role of Panx3 in the VNO of a Panx1 knock out mouse model. Front Mol Neurosci 11:135
Lohman AW, Isakson BE (2014) Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett 588:1379–1388
Narahari AK, Kreutzberger AJ, Gaete PS, Chiu Y-H, Leonhardt SA, Medina CB, Jin X, Oleniacz PW, Kiessling V, Barrett PQ, Ravichandran KS, Yeager M, Contreras JE, Tamm LK and Bayliss DA (2021) ATP and large signaling metabolites flux through caspase-activated Pannexin 1 channels. eLife 10:e64787
Nielsen BS, Toft-Bertelsen TL, Lolansen SD, Anderson CL, Nielsen MS, Thompson RJ, MacAulay N (2020) Pannexin 1 activation and inhibition is permeant-selective. J Physiol 598:361–379
Chiu YH, Schappe MS, Desai BN, Bayliss DA (2018) Revisiting multimodal activation and channel properties of Pannexin 1. J Gen Physiol 150:19–39
Yang Y, Delalio LJ, Best AK, Macal E, Milstein J, Donnelly I, Miller AM, McBride M, Shu X, Koval M, Isakson BE, Johnstone SR (2020) Endothelial Pannexin 1 channels control inflammation by regulating intracellular calcium. J Immunol 204:2995–3007
DeLalio LJ, Masati E, Mendu S, Ruddiman CA, Yang Y, Johnstone SR, Milstein JA, Keller TCSt, Weaver RB, Guagliardo NA, Best AK, Ravichandran KS, Bayliss DA, Sequeira-Lopez MLS, Sonkusare SN, Shu XH, Desai B, Barrett PQ, Le TH, Gomez RA and Isakson BE, (2020) Pannexin 1 channels in renin-expressing cells influence renin secretion and blood pressure homeostasis. Kidney Int 98:630–644
Bialecki J, Werner A, Weilinger NL, Tucker CM, Vecchiarelli HA, Egana J, Mendizabal-Zubiaga J, Grandes P, Hill MN, Thompson RJ (2020) Suppression of presynaptic glutamate release by postsynaptic metabotropic NMDA receptor signalling to Pannexin-1. J Neurosci 40:729–742
Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G (2007) Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26:433–443
Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, Barron B, Walk SF, Ghesquière B, Krupnick AS, Lorenz U, Ravichandran KS (2020) Metabolites released from apoptotic cells act as tissue messengers. Nature 580:130–135
Penuela S, Bhalla R, Nag K, Laird DW (2009) Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell 20:4313–4323
Koval M (2006) Pathways and control of connexin oligomerization. Trends Cell Biol 16:159–166
Deng Z, He Z, Maksaev G, Bitter RM, Rau M, Fitzpatrick JAJ, Yuan P (2020) Cryo-EM structures of the ATP release channel pannexin 1. Nat Struct Mol Biol 27:373–381
Jin Q, Zhang B, Zheng X, Li N, Xu L, Xie Y, Song F, Bhat EA, Chen Y, Gao N, Guo J, Zhang X, Ye S (2020) Cryo-EM structures of human pannexin 1 channel. Cell Res 30:449–451
Michalski K, Syrjanen JL, Henze E, Kumpf J, Furukawa H, Kawate T (2020) The Cryo-EM structure of pannexin 1 reveals unique motifs for ion selection and inhibition. Elife. 9:e54670
Mou L, Ke M, Song M, Shan Y, Xiao Q, Liu Q, Li J, Sun K, Pu L, Guo L, Geng J, Wu J, Deng D (2020) Structural basis for gating mechanism of Pannexin 1 channel. Cell Res 30:452–454
Qu R, Dong L, Zhang J, Yu X, Wang L, Zhu S (2020) Cryo-EM structure of human heptameric Pannexin 1 channel. Cell Res 30:446–448
Ruan Z, Orozco IJ, Du J, Lu W (2020) Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature 584:646–651
DeLalio LJ, Keller AS, Chen J, Boyce AKJ, Artamonov MV, Askew-Page HR, Keller TCSt, Johnstone SR, Weaver RB, Good ME, Murphy SA, Best AK, Mintz EL, Penuela S, Greenwood IA, Machado RF, Somlyo AV, Swayne LA, Minshall RD, Isakson BE (2018) Interaction between pannexin 1 and caveolin-1 in smooth muscle can regulate blood pressure. Arterioscler Thromb Vasc Biol 38(2065):2078
Papanikolaou A, Papafotika A, Murphy C, Papamarcaki T, Tsolas O, Drab M, Kurzchalia TV, Kasper M, Christoforidis S (2005) Cholesterol-dependent lipid assemblies regulate the activity of the ecto-nucleotidase CD39. J Biol Chem 280:26406–26414
Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL (1997) Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest 99:2588–2601
Gilleron J, Fiorini C, Carette D, Avondet C, Falk MM, Segretain D, Pointis G (2008) Molecular reorganization of Cx43, Zo-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J Cell Sci 121:4069–4078
Solan JL, Lampe PD (2020) Src regulation of Cx43 phosphorylation and gap junction turnover. Biomolecules 10:1596
Keller AS, Diederich L, Panknin C, DeLalio LJ, Drake JC, Sherman R, Jackson EK, Yan Z, Kelm M, Cortese-Krott MM, Isakson BE (2017) Possible roles for ATP release from RBCs exclude the cAMP-mediated Panx1 pathway. Am J Physiol Cell Physiol 313:C593–C603
Epp AL, Ebert SN, Sanchez-Arias JC, Wicki-Stordeur LE, Boyce AKJ, Swayne LA (2019) A novel motif in the proximal C-terminus of Pannexin 1 regulates cell surface localization. Sci Rep 9:9721
DeLalio LJ, Billaud M, Ruddiman CA, Johnstone SR, Butcher JT, Wolpe AG, Jin X, Keller TCSt, Keller AS, Riviere T, Good ME, Best AK, Lohman AW, Swayne LA, Penuela S, Thompson RJ, Lampe PD, Yeager M, Isakson BE (2019) Constitutive SRC-mediated phosphorylation of pannexin 1 at tyrosine 198 occurs at the plasma membrane. J Biol Chem 294(6940):6956
Boyce AK, Kim MS, Wicki-Stordeur LE, Swayne LA (2015) ATP stimulates pannexin 1 internalization to endosomal compartments. Biochem J 470:319–330
Boyce AKJ, Swayne LA (2017) P2X7 receptor cross-talk regulates ATP-induced pannexin 1 internalization. Biochem J 474:2133–2144
Nouri-Nejad D, O’Donnell BL, Patil CS, Sanchez-Pupo RE, Johnston D, Sayedyahossein S, Jurcic K, Lau R, Gyenis L, Litchfield DW, Jackson MF, Gloor GB, Penuela S (2021) Pannexin 1 mutation found in melanoma tumor reduces phosphorylation, glycosylation, and trafficking of the channel-forming protein. Mol Biol Cell 32:376–390
Wang W, Qu R, Dou Q, Wu F, Wang W, Chen B, Mu J, Zhang Z, Zhao L, Zhou Z, Dong J, Zeng Y, Liu R, Du J, Zhu S, Li Q, He L, Jin L, Wang L, Sang Q (2021) Homozygous variants in PANX1 cause human oocyte death and female infertility. Eur J Hum Genet. https://doi.org/10.1038/s41431-020-00807-4
Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467:863–867
Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA (2012) Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J Biol Chem 287:11303–11311
Stomberski CT, Hess DT, Stamler JS (2019) Protein S-nitrosylation: determinants of specificity and enzymatic regulation of S-nitrosothiol-based signaling. Antioxid Redox Signal 30:1331–1351
Lohman AW, Weaver JL, Billaud M, Sandilos JK, Griffiths R, Straub AC, Penuela S, Leitinger N, Laird DW, Bayliss DA, Isakson BE (2012) S-nitrosylation inhibits pannexin 1 channel function. J Biol Chem 287:39602–39612
Lohman AW, Weilinger NL, Santos SM, Bialecki J, Werner AC, Anderson CL, Thompson RJ (2019) Regulation of pannexin channels in the central nervous system by Src family kinases. Neurosci Lett 695:65–70
Yang Y, Delalio LJ, Best AK, Macal E, Milstein J, Donnelly I, Miller AM, McBride M, Shu X, Koval M, Isakson BE, Johnstone SR (2020) Endothelial pannexin 1 channels control inflammation by regulating intracellular calcium. J Immunol 204:2995–3007
Weilinger NL, Lohman AW, Rakai BD, Ma EM, Bialecki J, Maslieieva V, Rilea T, Bandet MV, Ikuta NT, Scott L, Colicos MA, Teskey GC, Winship IR, Thompson RJ (2016) Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci 19:432–442
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo J 25:5071–5082
Wang J, Ma M, Locovei S, Keane RW, Dahl G (2007) Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol 293:C1112–C1119
Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E (2008) P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 295:C752–C760
Silverman W, Locovei S, Dahl G (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 295:C761–C767
Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, Ravichandran KS (2014) Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507:329–334
Sandilos JK, Y-hH Chiu, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA (2012) Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C terminal autoinhibitory region. J Biol Chem 287:11303–11311
Isakson BE, Damon DN, Day KH, Liao Y, Duling BR (2006) Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol 290:H1199–H1205
Freitas-Andrade M, Bechberger JF, MacVicar BA, Viau V, Naus CC (2017) Pannexin1 knockout and blockade reduces ischemic stroke injury in female, but not in male mice. Oncotarget 8:36973–36983
Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR (2006) Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med 12:950–954
Yuan D, Wang Q, Wu D, Yu M, Zhang S, Li L, Tao L, Harris AL (2012) Monocyte-endothelial adhesion is modulated by Cx43-stimulated ATP release from monocytes. Biochem Biophys Res Commun 420:536–541
Huang G, Bao J, Shao X, Zhou W, Wu B, Ni Z, Wang L (2020) Inhibiting pannexin-1 alleviates sepsis-induced acute kidney injury via decreasing NLRP3 inflammasome activation and cell apoptosis. Life Sci 254:117791
Chen W, Zhu S, Wang Y, Li J, Qiang X, Zhao X, Yang H, D’Angelo J, Becker L, Wang P, Tracey KJ, Wang H (2019) Enhanced macrophage pannexin 1 expression and hemichannel activation exacerbates lethal experimental sepsis. Sci Rep 9:160
Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G (2015) Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43:923–932
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion—from mechanism to translation. Nat Med 17:1391–1401
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357:1121–1135
Kalogeris T, Baines CP, Krenz M and Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317
Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, Robbins MK, Kron IL (2002) Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg 73:1041–1048
Park SW, Kim M, Brown KM, D’Agati VD, Lee HT (2011) Paneth cell-derived interleukin-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology 53:1662–1675
Klausner JM, Paterson IS, Mannick JA, Valeri R, Shepro D, Hechtman HB (1989) Reperfusion pulmonary edema. Jama 261:1030–1035
Twombley K, Gattineni J, Bobulescu IA, Dwarakanath V, Baum M (2010) Effect of metabolic acidosis on neonatal proximal tubule acidification. Am J Physiol Regul Integr Comp Physiol 299:R1360–R1368
Sanada S, Komuro I, Kitakaze M (2011) Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol 301:H1723–H1741
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2016) Ischemia/reperfusion. Compr Physiol 7:113–170
Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R (2005) Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci 21:3277–3290
Vogt A, Hormuzdi SG, Monyer H (2005) Pannexin1 and pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res 141:113–120
Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R (2007) Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 146:9–16
Boassa D, Nguyen P, Hu J, Ellisman MH, Sosinsky GE (2014) Pannexin2 oligomers localize in the membranes of endosomal vesicles in mammalian cells while Pannexin1 channels traffic to the plasma membrane. Front Cell Neurosci 8:468
Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, Antle MC, Zamponi GW, Cahill CM, Borgland SL, De Koninck Y, Trang T (2017) Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med 23:355–360
Burns AR, Phillips SC, Sokoya EM (2012) Pannexin protein expression in the rat middle cerebral artery. J Vasc Res 49:101–110
Thompson RJ, Zhou N, MacVicar BA (2006) Ischemia opens neuronal gap junction hemichannels. Science 312:924–927
Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA (2008) Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322:1555–1559
Lipton SA, Rosenberg PA (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330:613–622
Kawamura M Jr, Ruskin DN, Masino SA (2010) Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci 30:3886–3895
Xiong XX, Gu LJ, Shen J, Kang XH, Zheng YY, Yue SB, Zhu SM (2014) Probenecid protects against transient focal cerebral ischemic injury by inhibiting HMGB1 release and attenuating AQP4 expression in mice. Neurochem Res 39:216–224
Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G (2009) The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 284:18143–18151
Cisneros-Mejorado A, Gottlieb M, Cavaliere F, Magnus T, Koch-Nolte F, Scemes E, Perez-Samartin A, Matute C (2015) Blockade of P2X7 receptors or pannexin-1 channels similarly attenuates postischemic damage. J Cereb Blood Flow Metab 35:843–850
Chen SP, Qin T, Seidel JL, Zheng Y, Eikermann M, Ferrari MD, van den Maagdenberg A, Moskowitz MA, Ayata C, Eikermann-Haerter K (2017) Inhibition of the P2X7-PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain 140:1643–1656
Seo JH, Dalal MS, Calderon F, Contreras JE (2020) Myeloid Pannexin-1 mediates acute leukocyte infiltration and leads to worse outcomes after brain trauma. J Neuroinflammation 17:245
Zou J, Yang J, Zhu X, Zhong J, Elshaer A, Matsusaka T, Pastan I, Haase VH, Yang HC, Fogo AB (2021) Stabilization of hypoxia-inducible factor ameliorates glomerular injury sensitization after tubulointerstitial injury. Kidney Int 99:620–631
Rosin DL, Okusa MD (2011) Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22:416–425
Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J (2012) Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol 303:F1454–F1459
DeLalio LJ, Masati E, Mendu S, Ruddiman CA, Yang Y, Johnstone SR, Milstein JA, Keller TCSt, Weaver RB, Guagliardo NA, Best AK, Ravichandran KS, Bayliss DA, Sequeira-Lopez MLS, Sonkusare SN, Shu XH, Desai B, Barrett PQ, Le TH, Gomez RA, Isakson BE (2020) Pannexin 1 channels in renin-expressing cells influence renin secretion and blood pressure homeostasis. Kidney Int 98(630):644
Su L, Jiang X, Yang C, Zhang J, Chen B, Li Y, Yao S, Xie Q, Gomez H, Murugan R, Peng Z (2019) Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J Biol Chem 294:19395–19404
Kristiansen SB, Skovsted GF, Berchtold LA, Radziwon-Balicka A, Dreisig K, Edvinsson L, Sheykhzade M, Haanes KA (2018) Role of pannexin and adenosine triphosphate (ATP) following myocardial ischemia/reperfusion. Scand Cardiovasc J 52:340–343
Dolmatova E, Spagnol G, Boassa D, Baum JR, Keith K, Ambrosi C, Kontaridis MI, Sorgen PL, Sosinsky GE, Duffy HS (2012) Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol 303:H1208–H1218
Vessey DA, Li L, Kelley M (2011) P2X7 receptor agonists pre- and postcondition the heart against ischemia-reperfusion injury by opening pannexin-1/P2X(7) channels. Am J Physiol Heart Circ Physiol 301:H881–H887
Molica F, Meens MJ, Dubrot J, Ehrlich A, Roth CL, Morel S, Pelli G, Vinet L, Braunersreuther V, Ratib O, Chanson M, Hugues S, Scemes E, Kwak BR (2017) Pannexin1 links lymphatic function to lipid metabolism and atherosclerosis. Sci Rep 7:13706
Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA (2012) Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18:600–604
Lutz SE, Gonzalez-Fernandez E, Ventura JC, Perez-Samartin A, Tarassishin L, Negoro H, Patel NK, Suadicani SO, Lee SC, Matute C, Scemes E (2013) Contribution of pannexin1 to experimental autoimmune encephalomyelitis. PLoS One 8:e66657
Acknowledgements
We thank Abigail Wolpe and Scott Johnstone for critical feedback, and Anita Impagliazzo for the illustrations.
Funding
This work was supported by HL137112 (MK and BEI) and HL120840 (BEI).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
No experimental data was generated for this review.
Informed consent
No experimental data was generated for this review.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on The contribution of pannexin-1, connexins and CALHM ATP-release channels to purinergic signalling — Guest Editor Charles Kennedy
Rights and permissions
About this article
Cite this article
Koval, M., Cwiek, A., Carr, T. et al. Pannexin 1 as a driver of inflammation and ischemia–reperfusion injury. Purinergic Signalling 17, 521–531 (2021). https://doi.org/10.1007/s11302-021-09804-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-021-09804-8