Abstract
Introduction
Breast cancer (BC) is positioned as the second among all cancers remaining at the top of women´s diseases worldwide followed by colorectum, lung, cervix, and thyroid cancers. The main drawback of most the screening/diagnostic methods is their low sensitivity/specificity and in some cases the invasive procedure required to obtain the samples.
Objectives
On the present investigation, we report a statistical design was to evaluate by central composite design the influence towards the optimization of the most significant variables of solid-phase microextraction (SPME) procedure for the isolation of volatile organic metabolites (VOMs) from urine of BC patients (N = 31) and healthy individuals (CTL; N = 40). The establishment of the urinary volatomic composition, through gas chromatography-mass spectrometry (GC–MS) analysis, can boost the identification of volatile organic metabolites (VOMs) potential BC biomarkers useful to be used together or to complement the current BC diagnostics tools. Better early detection methods are needed to improve the outcomes of patients with BC.
Methods
Several combinations of experiments were considered with a central composite design (CCD) of response surface methodology (RSM) for the urinary volatomic pattern. Three-level three-factor CCD was employed assessing the most important extraction-influencing variables—fiber coating, NaCl amount, extraction time and temperature. The optimal conditions were achieved using a carboxen/polydimethylsiloxane fiber with 15% (w/v) NaCl during 75 min at 50 °C.
Results
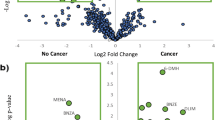
A total of ten VOMs belonging to sulfur compounds, terpenoids and carbonyl compounds presented the highest contribution towards discrimination of BC patients from CTL (variable importance in projection (VIP) > 1, p < 0.05). The discrimination efficiency and accuracy of urinary metabolites was ascertained by receiver operating characteristic (ROC) curve analysis that allowed the identification of some metabolites with highest sensitivity and specificity to discriminate the groups.
Conclusions
The results obtained with this approach suggest the possibility to identify endogenous metabolites as a platform to discovery potential BC biomarkers and paves a way to explore the related metabolomic pathways in order to improve BC diagnostic tools.





Similar content being viewed by others
References
Ahmed, I., Greenwood, R., Costello, B., Ratcliffe, N., & Probert, C. S. (2016). Investigation of faecal volatile organic metabolites as novel diagnostic biomarkers in inflammatory bowel disease. Alimentary Pharmacology & Therapeutics, 43(5), 596–611.
Baker, S. G. (2003). The central role of receiver operating characteristic (ROC) curves in evaluating tests for the early detection of cancer. JNCI Journal of the National Cancer Institute, 95(7), 511–515.
Beger, R. D. (2013). A review of applications of metabolomics in cancer. Metabolites, 3(3), 552–574.
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424.
Bylesjö, M., Rantalainen, M., Cloarec, O., Nicholson, J. K., Holmes, E., & Trygg, J. (2006). OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. Journal of Chemometrics, 20(8–10), 341–351.
Calejo, I., Moreira, N., Araújo, A. M., Carvalho, M., de Bastos, M. L., & de Pinho, P. G. (2016). Optimisation and validation of a HS-SPME–GC–IT/MS method for analysis of carbonyl volatile compounds as biomarkers in human urine: Application in a pilot study to discriminate individuals with smoking habits. Talanta, 148, 486–493.
Cavaco, C., Pereira, J. A. M., Taunk, K., Taware, R., Rapole, S., Nagarajaram, H., et al. (2018). Screening of salivary volatiles for putative breast cancer discrimination: An exploratory study involving geographically distant populations. Analytical and Bioanalytical Chemistry, 410(18), 1–10. https://doi.org/10.1007/s00216-018-1103-x.
Denkert, C., Bucher, E., Hilvo, M., Salek, R., Orešič, M., Griffin, J., et al. (2012). Metabolomics of human breast cancer: New approaches for tumor typing and biomarker discovery. Genome Medicine, 4(4), 37. https://doi.org/10.1186/gm336.
Dougan, M. M., Li, Y., Chu, L. W., Haile, R. W., Whittemore, A. S., Han, S. S., et al. (2018). Metabolomic profiles in breast cancer: A pilot case-control study in the breast cancer family registry. BMC Cancer, 18(1), 532. https://doi.org/10.1186/s12885-018-4437-z.
Duffy, M. J. (2006). Serum tumor markers in breast cancer: Are they of clinical value? Clinical Chemistry, 52(3), 345–351.
Filipiak, W., Filipiak, A., Sponring, A., Schmid, T., Zelger, B., Ager, C., et al. (2014). Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. Journal of Breath Research, 8(2), 027111.
Hajian-Tilaki, K. (2013). Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian Journal of Internal Medicine, 4(2), 627–635.
Janitza, S., & Hornung, R. (2018). On the overestimation of random forest’s out-of-bag error. PLoS ONE, 13(8), e0201904. https://doi.org/10.1371/journal.pone.0201904.
Koek, M. M., Jellema, R. H., van der Greef, J., Tas, A. C., & Hankemeier, T. (2011). Quantitative metabolomics based on gas chromatography mass spectrometry: Status and perspectives. Metabolomics: Official journal of the Metabolomic Society, 7(3), 307–328.
Lavra, L., Catini, A., Ulivieri, A., Capuano, R., Baghernajad Salehi, L., Sciacchitano, S., et al. (2015). Investigation of VOCs associated with different characteristics of breast cancer cells. Scientific Reports, 5, 13246.
McCartney, A., Vignoli, A., Biganzoli, L., Love, R., Tenori, L., Luchinat, C., et al. (2018). Metabolomics in breast cancer: A decade in review. Cancer Treatment Reviews, 67, 88–96.
Milosevic, M., Jankovic, D., Milenkovic, A., & Stojanov, D. (2018). Early diagnosis and detection of breast cancer. Technology and Health Care, 26(4), 729–759.
Monteiro, M. S., Carvalho, M., de Lourdes Bastos, M., & de Pinho, P. G. (2014b). Biomarkers in renal cell carcinoma: A metabolomics approach. Metabolomics. https://doi.org/10.1007/s11306-014-0659-5.
Monteiro, M., Carvalho, M., Henrique, R., Jerónimo, C., Moreira, N., de Lourdes Bastos, M., et al. (2014a). Analysis of volatile human urinary metabolome by solid-phase microextraction in combination with gas chromatography–mass spectrometry for biomarker discovery: Application in a pilot study to discriminate patients with renal cell carcinoma. European Journal of Cancer, 50(11), 1993–2002.
Musteata, F. M., & Pawliszyn, J. (2007). Bioanalytical applications of solid-phase microextraction. TrAC, Trends in Analytical Chemistry, 26(1), 36–45.
Poli, D., Carbognani, P., Corradi, M., Goldoni, M., Acampa, O., Balbi, B., et al. (2005). Exhaled volatile organic compounds in patients with non-small cell lung cancer: Cross sectional and nested short-term follow-up study. Respiratory Research, 6, 71.
Porto-Figueira, P., Pereira, J. A. M., & Câmara, J. S. (2018). Exploring the potential of needle trap microextraction combined with chromatographic and statistical data to discriminate different types of cancer based on urinary volatomic biosignature. Analytica Chimica Acta, 1023, 53–63. https://doi.org/10.1016/j.aca.2018.04.027.
Raman, M., Ahmed, I., Gillevet, P. M., Probert, C. S., Ratcliffe, N. M., Smith, S., et al. (2013). Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology, 11(7), 868–875.
Silva, C. L., Passos, M., & Câmara, J. S. (2011). Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. British Journal of Cancer, 105(12), 1894–1904.
Silva, C. L., Passos, M., & Câmara, J. S. (2012). Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers—a powerful strategy for breast cancer diagnosis. Talanta, 89, 360–368.
Silva, C. L., Perestrelo, R., Silva, P., Tomás, H., & Câmara, J. S. (2017). Volatile metabolomic signature of human breast cancer cell lines. Scientific Reports, 7, 43969.
Slupsky, C. M., Steed, H., Wells, T. H., Dabbs, K., Schepansky, A., Capstick, V., et al. (2010). Urine metabolite analysis offers potential early diagnosis of ovarian and breast cancers. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 16(23), 5835–5841.
Sun, Y., Liu, J., & Kennedy, J. F. (2010). Application of response surface methodology for optimization of polysaccharides production parameters from the roots of Codonopsis pilosula by a central composite design. Carbohydrate Polymers, 80(3), 949–953.
Xia, J., Broadhurst, D. I., Wilson, M., & Wishart, D. S. (2013). Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics, 9(2), 280–299.
Xia, J., Wishart, D. S., Xia, J., & Wishart, D. S. (2016). Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Current Protocols in Bioinformatics (pp. 14.10.1–14.10.91). Hoboken, NJ: John Wiley & Sons Inc.
Zhang, A., Sun, H., Qiu, S., & Wang, X. (2013). Metabolomics in noninvasive breast cancer. Clinica Chimica Acta International Journal of Clinical Chemistry, 424, 3–7.
Zhang, A., Sun, H., Wang, P., Han, Y., & Wang, X. (2012). Recent and potential developments of biofluid analyses in metabolomics. Journal of Proteomics, 75(4), 1079–1088.
Acknowledgements
This work was supported by FCT-Fundação para a Ciência e a Tecnologia (projects PEstOE/QUI/UI0674/2019, CQM, Portuguese Government funds, and INNOINDIGO/0001/2015), Madeira 14-20 Program (project PROEQUIPRAM-Reforço do Investimento em Equipamentos e Infraestruturas Científicas na RAM-M1420-01-0145-FEDER-000008) and by ARDITI-Agência Regional para o Desenvolvimento da Investigação Tecnologia e Inovação (project M1420-01-0145-FEDER-000005-Centro de Química da Madeira-CQM + (Madeira 14-20)). The authors also acknowledge the FCT for the Ph.D grant SFRH/BD/97039/2013 (Catarina L. Silva) and the PostDoctoral fellowship SFRH/BPD/97387/2013 (Rosa Perestrelo). Pedro Silva acknowledges ARDITI for the Ph.D Grant under the M1420 Project—09-5369-FSE-000001.
Author information
Authors and Affiliations
Contributions
CS: Conceived and designed the research, performed BC and CTL urine extractions, analysis by HS-SPME/GC–qMS, data analysis and wrote the paper. RP: performed the design of experiments and data analysis. PS: performed the design of experiments, statistical analysis and interpretation. HT: co-supervisor of the work, conception of study, experimental design, and manuscript preparation. JSC: main supervisor of the work, conceived and designed the study, experimental design, and manuscript preparation. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silva, C.L., Perestrelo, R., Silva, P. et al. Implementing a central composite design for the optimization of solid phase microextraction to establish the urinary volatomic expression: a first approach for breast cancer. Metabolomics 15, 64 (2019). https://doi.org/10.1007/s11306-019-1525-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-019-1525-2