Abstract
Introduction
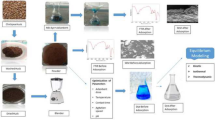
A biosorbent was developed by simple dried Agaricus bisporus (SDAB) and effectively used for the biosorption of cationic dyes, Crystal Violet and Brilliant Green.
Materials and methods
For the evaluation of the biosorbent system, all the batch equilibrium parameters like pH, biomass dose, contact time, and temperature were optimized to determine the decolorization efficiency of the biosorbent. The maximum yields of dye removal were achieved at pH 4.0 for Crystal Violet (CV) and pH 5.0 for Brilliant Green (BG), which are closer to their natural pH also.
Result and discussion
Equilibrium was established at 60 and 40 min for CV and BG, respectively. Pseudo first-order, pseudo second-order, and intraparticle-diffusion kinetic models were studied at different temperatures. Isotherm models such as Freundlich, Langmuir, and Dubinin–Radushkevich were also studied. Biosorption processes were successfully described by Langmuir isotherm model and the pseudo second-order kinetic model.
Conclusions
The biosorption capacity of A. bisporus over CV and BG were found as 21.74 and 12.16 mg gm−1. Thermodynamic parameters indicated that the CV and BG dye adsorption onto A. bisporus is spontaneous and exothermic in the single and ternary systems. Scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectroscopy were used for the surface morphology, crystalline structure of biosorbent, and dye–biosorbent interaction, respectively. This analysis of the biosorption data confirmed that these biosorption processes are ecofriendly and economical. Thus, this biomass system may be useful for the removal of contaminating cationic dyes.







Similar content being viewed by others
References
Akar T, Anilan B, Kaynak Z, Gorgulu A, Akar ST (2008) Batch and dynamic flow biosorption potential of Agaricus bisporus/Thuja orientalis biomass mixture for decolorization of RR45 dye. Ind Eng Chem Res 47:9715–9723
Akara ST, Asli G, Zerrin K, Burcu A, Tamer A (2009) Biosorption of reactive blue 49 dye under batch and continuous mode using a mixed biosorbent of macro-fungus Agaricus bisporus and Thuja orientalis cones. Chem Eng J 148:26–34
Aksu Z, Dönmez GA (2003) comparative study on the biosorption characteristics of some yeast for Remazol Blue reactive dye. Chemosphere 50:1075–1083
Aksu Z, Tatlı AI, Tunc O (2008) A comparative adsorption/biosorption study of acid blue 161: effect of temperature on equilibrium and kinetic parameters. Chem Eng J 142:23–39
Annadurai G, Juang RS, Lee DJ (2002) Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater 92:263–274
Botero AEC, Torem ML, de Mesquita LMS (2008) Surface chemistry fundamentals of biosorption of Rhodococcus opacus and its effect in calcite and magnesite flotation. Miner Eng 21:83–92
Chen L, Lu L, Shao W, Lu F (2011) Kinetics and equilibria of Cd(II) adsorption onto a chemically modified lawny grass with H[BTMPP]. J Chem Eng Data 56:1059–1068
Chowdhury S, Saha P (2010) Sea shell powder as a new adsorbent to remove basic green 4 (malachite green) from aqueous solutions: equilibrium, kinetic and thermodynamic studies. Chem Eng J 164:168–177
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168
Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. J Hazard Mater 167:1–9
Ergene A, Ada K, Tan S, Katırcıoğlu H (2009) Removal of Remazol Brilliant Blue R dye from aqueous solutions by adsorption onto immobilized Scenedesmus quadricauda: equilibrium and kinetic modeling studies. Desalination 249:1308–1314
Ertugay N, Bayhan YK (2010) The removal of copper (II) ion by using mushroom biomass (Agaricus bisporus) and kinetic modeling. Desalination 255:137–142
Fry BA (1957) Basic triphenylmethane dyes and the inhibition of glutamine synthesis by Staphylococcus aureus. J Gen Microbiol 16:341–349
Fu Y, Viraraghavan T (2001) Fungal decolorization of dye wastewaters: a review. Bioresour Technol 79:251–262
Fu Y, Virarahavan T (2000) Removal of a dye from an aqueous solution by the fungus Aspergillus niger. Water Qual Res J Can 35:95–111
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28
Ghaedi M, Hossainian H, Montazerozohori M, Shokrollahi A, Shojaipour F, Soylak M, Purkait MK (2011) A novel acorn based adsorbent for the removal of brilliant green. Desalination 281:226–233
Gupta VK, Ali I (2008) Removal of endosulfan and methoxychlor from water on carbon slurry. Environ Sci Technol 42:766–770
Gupta VK, Rastogi A (2008a) Biosorption of lead (II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—a comparative study. Colloids Surf B: Biointerfaces 64:170–178
Gupta VK, Rastogi A (2008b) Equilibrium and kinetic modelling of cadmium (II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Rastogi A (2008c) Sorption and desorption studies of chromium (VI) from nonviable cyanobacterium Nostoc muscorum biomass. J Hazard Mater 154:347–354
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal: a review. J Environ Manage 90:2313–2342
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997) Process development for the removal of zinc and cadmium from wastewater using slag—a Blast furnace waste material. Sep Sci Technol 32:2883–2912
Gupta VK, Srivastava SK, Tyagi R (2000) Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material). Water Res 34:1543–1550
Gupta VK, Ali I, Saini VK, Gerven TV, der Bruggen BV, Vandecasteele C (2005) Removal of dyes from wastewater using bottom ash. Ind Eng Chem Res 44:3655–3664
Gupta VK, Mittal A, Jain R, Mathur M, Sikarwar S (2006a) Adsorption of safranin-T from wastewater using waste materials-activated carbon and activated rice husks. J Colloid Interface Sci 303:80–86
Gupta VK, Alok M, Gajbe V, Mittal J (2006b) Removal and recovery of the hazardous Azo Dye Acid Orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Ali I, Saini VK (2007a) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315:87–93
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007b) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interface Sci 309:464–469
Gupta VK, Jain R, Varshney S (2007c) Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk—an agricultural waste. J Hazard Mater 142:443–448
Gupta VK, Mittal A, Gajbe V, Mittal J (2008) Adsorption of basic fuchsin using waste materials—bottom ash and deoiled soya—as adsorbents. J Colloid Interface Sci 319:30–39
Gupta VK, Rastogi A, Arunima N (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Gupta VK, Nayak A, Agarwal S, Shrivastava M (2011) Removal of the hazardous dye—Tartrazine by photodegradation on titanium dioxide surface. Mater Sci Eng C 31:1062–1067
Hameed BH (2009) Spent tea leaves: a new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. J Hazard Mater 161:753–759
Hazrat A (2010) Biodegradation of synthetic dyes—a review. Water Air Soil Pollut 213:251–273
Imran A, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc. doi:10.1038/nprot.2006.370 1:2661-266
Khouni I, Marrot B, Moulin P, Amar RB (2011) Decolourization of the reconstituted textile effluent by different process treatments: enzymatic catalysis, coagulation/flocculation and nanofiltration processes. Desalination 268:27–37
Kismir Y, Aroguz AZ (2011) Adsorption characteristics of the hazardous dye brilliant green on saklıkent mud. Chem Eng J 172:199–206
Maurya NS, Mittal AK, Cornel P, Rother E (2006) Biosorption of dyes using dead macro fungi: effect of dye structure, ionic strength and pH. Bioresour Technol 97:512–521
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010a) Adsorption of hazardous crystal violet from wastewater by waste materials. J Colloid Interface Sci 343:463–473
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010b) Decoloration treatment of a hazardous triarylmethane dye, light green SF (yellowish) by waste material adsorbents. J Colloid Interface Sci 342:518–527
Mohamad A, Mohd S, Mahmoud DK, Wan A, Wan AK, Azni I (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280:1–13
Monash P, Pugazhenthi G (2009) Adsorption of crystal violet dye from aqueous solution using mesoporous materials synthesized at room temperature. Adsorption 15:390–405
Muhammad JI, Muhammad NA (2007) Thermodynamics of adsorption of dyes from aqueous media on activated charcoal. Bahauddin Zakariya University, Multan, Pakistan. J Res Sci 18:91–99
Namasivayam C, Kavitha D (2002) Removal of Congo red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigments 54:47–58
Namasivayam C, Prabha D, Kumutha M (1998) Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresour Technol 64:77–79
Nandi BK, Goswami A, Purkait MK (2009) Adsorption characteristics of brilliant green dye on kaolin. J Hazard Mater 161:387–395
Olsson M (2006) Wheat straw and peat for fuel pellets—organic compounds from combustion. Biomass Bioenergy 30:555–564
Onal Y (2006) Kinetics of adsorption of dyes from aqueous solution using activated carbon prepared from waste apricot. J Hazard Mater 137:1719–1728
Parshetti GK, Parshetti SG, Telke AA, Kalyani DC, Doong RA, Govindwar SP (2011) Biodegradation of crystal violet by Agrobacterium radiobacter. J Environ Sci 23:1384–1393
Pavan FA, Lima EC, Dias SLP, Mazzocato AC (2008) Methylene blue biosorption from aqueous solutions by yellow passion fruit waste. J Hazard Mater 150:703–712
Rodríguez A, García J, Ovejero G, Mestanza M (2009) Adsorption of anionic and cationic dyes on activated carbon from aqueous solutions: equilibrium and kinetics. J Hazard Mater 172:1311–1320
Saeed A, Sharif M, Iqbal M (2010) Application potential of grapefruit peel as dye sorbent: kinetics, equilibrium and mechanism of crystal violet adsorption. J Hazard Mater 179:564–572
Saha P, Chowdhury S, Gupta S, Kumar I (2010) Insight into adsorption equilibrium, kinetics and thermodynamics of malachite green onto clayey soil of Indian origin. Chem Eng J 165:874–882
Saha PD, Chakraborty S, Chowdhury S (2012) Batch and continuous (fixed-bed column) biosorption of crystal violet by Artocarpus heterophyllus (jackfruit) leaf powder. Colloids Surf B: Biointerfaces 92:262–270
Sethuraman P, Balasubramanian N (2010) Removal of Cr(VI) from aqueous solution using Bacillus subtilis, Pseudomonas aeruginosa and Enterobacter cloacae. Int J Eng Sci Technol 2:1811–1825
Sun XF, Wang SG, Liu XW, Gong WX, Bao N, Gao BY, Zhang HY (2008) Biosorption of malachite green from aqueous solutions onto aerobic granules: kinetic and equilibrium studies. Bioresour Technol 99:3475–3483
Tsai WT, Chang CY, Lin MC, Chien SF, Sun HF, Hsieh MF (2001) Adsorption of acid dye onto activated carbons prepared from agricultural waste bagasse by ZnCl2 activation. Chemosphere 45:51–58
Wang JL, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–60
Yang BL, Goto M, Goto S (1989) Affinity separation by the combined process of batch adsorption and fixed bed elution. Colloids Surfaces 3:369–378
Acknowledgments
The authors like to thank the Department of Industrial Chemistry, Alagappa University, Karaikudi, India and IITR, Lucknow, India for its support and providing the facilities for this work. One of the authors Priyanka Pandey gratefully acknowledges the Department of Science & Technology, New Delhi for DST-PURSE, Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Pandey, P., Singh, R.P., Singh, K.N. et al. Evaluation of the individuality of white rot macro fungus for the decolorization of synthetic dye. Environ Sci Pollut Res 20, 238–249 (2013). https://doi.org/10.1007/s11356-012-0875-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0875-3