Abstract
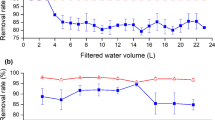
Activated carbon was prepared from date pits via chemical activation with H3PO4. The effects of activating agent concentration and activation temperature on the yield and surface area were studied. The optimal activated carbon was prepared at 450 °C using 55 % H3PO4. The prepared activated carbon was characterized by Fourier transform infrared spectroscopy, scanning electron microscopy, thermogravimetric-differential thermal analysis, and Brunauer, Emmett, and Teller (BET) surface area. The prepared date pit-based activated carbon (DAC) was used for the removal of bromate (BrO3 −). The concentration of BrO3 − was determined by ultra-performance liquid chromatography-mass tandem spectrometry (UPLC-MS/MS). The experimental equilibrium data for BrO3 − adsorption onto DAC was well fitted to the Langmuir isotherm model and showed maximum monolayer adsorption capacity of 25.64 mg g−1. The adsorption kinetics of BrO3 − adsorption was very well represented by the pseudo-first-order equation. The analytical application of DAC for the analysis of real water samples was studied with very promising results.






Similar content being viewed by others
References
Ahmed MJ, Theydan SK (2012) Physical and chemical characteristics of activated carbon prepared by pyrolysis of chemically treated date pits and its ability to adsorb organics. Powder Technol 229:237–245
Alhamed YA (2009) Adsorption kinetics and performance of packed bed adsorber for phenolremoval using activated carbon from dates’ pits. J Hazard Mater 170:763–770
Alhamed YA, Bamufleh HS (2009) Sulfur removal from model diesel fuel using granular activated carbon from dates’ pits activated by ZnCl2. Fuel 88:87–94
ALOthman ZA, Ali R, Naushad M (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:238–247
ALOthman ZA, Naushad M, Ali R (2013) Kinetic, equilibrium isotherm and thermodynamic studies of Cr(VI) adsorption onto low-cost adsorbent developed from peanut shell activated with phosphoric acid. Environ Sci Pollut Res 20:3351–3365
Alsohaimi IH, Alothman ZA, Khan MR, Abdalla MA, Busquets R, Alomary AK (2012) Determination of bromate in drinking water by ultra–performance liquid chromatography–tandem mass spectrometry. J Sep Sci 35:2538–2543
Barrett EP, Joyner LC, Halenda PH (1951) The determination of pore volume and area distribution in porous substances. J Am Chem Soc 73:373–380
Bhatnagar A, Sillanpaa M (2012) Sorption studies of bromate removal from water by nano-Al2O3. Sep Sci Technol 47:89–95
Bhatnagar A, Choi YH, Yoon YJ, Shin Y, Jeon BH, Kang JW (2009) Bromate removal from water by granular ferric hydroxide (GFH). J Hazard Mater 170:134–140
Bonacquisti TP (2006) A drinking water utility’s perspective on bromide, bromate, and ozonation. Toxicology 221:145–148
Bouchelta C, Medjram MS, Bertrand O, Bellat JP (2008) Preparation and characterization of activated carbon from date pits by physical activation with steam. J Anal Appl Pyrolysis 82:70–77
Bouhamed F, Elouear Z, Bouzid J (2012) Adsorptive removal of copper(II) from aqueous solutions on activated carbon prepared from Tunisian date stones: equilibrium, kinetics and thermodynamics. J Taiwan Inst Chem Eng 43:741–749
Butler R, Godley A, Lytton L, Cartmell E (2005) Bromate environmental contamination: review of impact and possible treatment. Crit Rev Environ Sci Technol 35:193–217
Campbell KC (2006) Bromate-induced ototoxicity. Toxicology 221:205–211
Chen H, Xu Z, Wan H, Zheng J, Yin D, Zheng S (2010) Aqueous bromate reduction by catalytic hydrogenation over Pd/Al2O3 catalysts. Appl Catal B Environ 96:307–313
Chen R, Yang Q, Zhong Y, Li X, Liu Y, Li X-M, Du W-X, Zeng G-M (2014) Sorption of trace levels of bromate by macroporous strong base anion exchange resin: influencing factors, equilibrium isotherms and thermodynamic studies. Desalination 344:306–312
Chitrakar R, Tezuka S, Sonoda A, Sakane K, Hirotsu T (2009) Bromate ion-exchange properties of crystalline akaganeite. Ind Eng Chem Res 48:2107–2112
Chitrakar R, Makita Y, Sonoda A, Hirotsu T (2011) Adsorption of trace levels of bromate from aqueous solution by organo-montmorillonite. Appl Clay Sci 51:375–379
Danish M, Hashim R, Ibrahim MNM, Sulaiman O (2014) Optimized preparation for large surface area activated carbon from date (Phoenix dactylifera L.) stone biomass. Biomass Bioenergy 61:167–178
Duranoglu D, Trochimczuk AW, Bekera UA (2010) Comparison study of peach stone and acrylonitrile-divinylbenzene copolymer based activated carbons as chromium(VI) sorbents. Chem Eng J 165:56–63
Erlich C, Bjornbom E, Bolado D, Giner M, Fransson TH (2006) Pyrolysis and gasification of pellets from sugar cane bagasse and wood. Fuel 85:1535–1540
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Ganesan P, Kamaraj R, Vasudevan S (2013a) Application of isotherm, kinetic and thermodynamic models for the adsorption of nitrate ions on graphene from aqueous solution. J Taiwan Inst Chem Eng 44:808–814
Ganesan P, Lakshmi J, Sozhan G, Vasudevan S (2013b) Removal of manganese from water by electrocoagulation: adsorption, kinetics and thermodynamic studies. Can J Chem Eng 91:448–458
Gao Y, Yue Q, Gao B, Sun Y, Wang W, Li Q, Wang Y (2013) Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni(II) adsorption. Chem Eng J 217:345–353
Gomez-Serrano V, Alvarez PM, Jaramillo J, Beltran FJ (2002) Formation of oxygen structures by ozonation of carbonaceous materials prepared from cherry stones: II. Kinetic study. Carbon 40:523–529
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity. Academic, New York
Guo Y, Straw DAR (2007) Physicochemical properties of carbons prepared from pecan shell by phosphoric acid activation. Bioresour Technol 98:1513–1521
Haimour NM, Emeish S (2006) Utilization of date stones for production of activated carbon using phosphoric acid. Waste Manag 26:651–660
Hamada JS, Hashim IB, Sharif FA (2002) Preliminary analysis and potential uses of date pits in foods. Food Chem 76:135–137
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Kamaraj R, Vasudevan S (2015) Decontamination of selenate from aqueous solution by oxidized multi-walled carbon nanotubes. Powder Technol 274:268–275
Kamaraj R, Davidson DJ, Sozhan G, Vasudevan S (2014) Adsorption of 2, 4-dichlorophenoxyacetic acid (2, 4-D) from water by in situ generated metal hydroxides using sacrificial anodes. J Taiwan Inst Chem Eng 45:2943–2949
Kamaraj R, Davidson DJ, Sozhan G, Vasudevan S (2015) Adsorption of herbicide 2-(2,4-dichlorophenoxy)propanoic acid by electrochemically generated aluminum hydroxides: an alternative to chemical dosing. RSC Adv 5:39799–39809
Khan MA, ALOthman ZA , Naushad M, Khan MR, Luqman M (2015) Adsorption of methylene blue on strongly basic anion exchange resin (Zerolit DMF): kinetic, isotherm, and thermodynamic studies. Environ Sci Pollut Res 53:515–523
Lagergren S (1898) About the theory of so called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar Band 24:1–39
Lakshmi J, Vasudevan S (2013) Graphene—a promising material for removal of perchlorate (ClO4 −) from water. Environ Sci Pollut Res 20:5114–5124
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lazaro MJ, Galvez ME, Artal S, Palacios JM, Moliner R (2007) Preparation of steam-activated carbons as catalyst supports. J Anal Appl Pyrolysis 78:301–315
Liou TH (2010) Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem Eng J 158:129–142
Marsh H, Rodriguez-Reinoso F (2006) Activated carbon. Elsevier, Amsterdam
Martinez ML, Torres MM, Guzman CA, Maestri DM (2006) Preparation and characteristics of activated carbon from olive stones and walnut shells. Ind Crop Prod 23:23–28
Merzougui Z, Addoun F (2008) Effect of oxidant treatment of date pit activated carbons application to the treatment of waters. Desalination 222:394–403
Molina-Sabio M, Rodríguez-Reinoso F (2004) Role of chemical activation in the development of carbon porosity. Colloids Surf A Physicochem Eng Asp 241:15–25
Molina-Sabio M, Rodríguez Reinoso F, Caturla F, Sellés MJ (1995) Porosity in granular carbons activated with phosphoric acid. Carbon 33:1105–1113
Naushad M, ALOthman ZA, Khan MR, Wabaidur SM (2013) Removal of bromate from water using de-acidite FF-IP resin and determination by ultra-performance liquid chromatography-tandem mass spectrometry. Clean Soil Air Water 41:528–533
Naushad M, Khan MR, ALOthman ZA, Awual MR (2015) Bromate removal from water samples using strongly basic anion exchange resin Amberlite IRA-400: kinetics, isotherms and thermodynamic studies. Desalin Water Treat. doi:10.1080/19443994.2015.1005157
Prahas D, Kartika Y, Indraswati N, Ismadji S (2008) Activated carbon from jackfruit peel waste by H3PO4 chemical activation: characterization of activated carbon prepared by phosphoric acid activation of olive pits, pore structure and surface chemistry characterization. Chem Eng J 140:32–42
Putun E, Uzun BB, Putun AE (2006) Fixed-bed catalytic pyrolysis of cotton-seed cake: effects of pyrolysis temperature, natural zeolite content and sweeping gas flow rate. Bioresour Technol 97:701–710
Reddy KSK, Al-Shoaibi A, Srinivasakannan C (2012) A comparison of microstructure and adsorption characteristics of activated carbons by CO2 and H3PO4 activation from date palm pits. New Carbon Mater 27:344–351
Reinoso FR, Molina-Sabio M, Gonzalez MT (1995) The use of steam and CO2 as activating agents in the preparation of activated carbons. Carbon 33:15–23
Rios RVRA, Martinez-Escandell M, Molina-Sabio M, Rodriguez-Reinoso F (2006) Carbon foam prepared by pyrolysis of olive stones under steam. Carbon 44:1448–1454
Salman JM, Njoku VO, Hameed BH (2011) Bentazon and carbofuran adsorption onto date seed activated carbon: kinetics and equilibrium. Chem Eng J 173:361–368
Sekirifa ML, Hadj-Mahammed M, Pallier S, Baameur L, Richard D, Al-Dujaili AH (2013) Preparation and characterization of an activated carbon from a date pits variety by physical activation with carbon dioxide. J Anal Appl Pyrolysis 99:155–160
Sentorun-Shalaby C, Ucak-Astarlıoglu MG, Artok L, Sarici C (2006) Preparation and characterization of activated carbons by one-step steam pyrolysis/activation from apricot stones. Microporous Mesoporous Mater 88:126–134
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Toles CA, Marshall WE, John MM, Wartelle LH, McAloon A (2000) Acid-activated carbons from almond shells: physical, chemical and adsorptive properties and estimated cost of production. Bioresour Technol 71:87–92
WHO (2011) Guideline for drinking-water quality, second addendum to fourth edition
Xu C, Shi J, Zhou W, Gao B, Yue Q, Wang X (2012) Bromate removal from aqueous solutions by nano crystalline akaganeite (β-FeOOH)-coated quartz sand (CACQS). Chem Eng J 187:63–68
Youssef AM, Radwan NRE, Abdel-Gawad I, Singer GAA (2005) Textural properties of activated carbons from apricot stones. Colloids Surf A Physicochem Eng Asp 252:143–151
Zeino A, Abulkibash A, Khaled M, Atieh M (2014) Bromate removal from water using doped iron nanoparticles on multiwalled carbon nanotubes (CNTS). J Nanomater 2014:1–9
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia (Award Number 12-WAT3138-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 161 kb)
Rights and permissions
About this article
Cite this article
Naushad, M., Khan, M.R., ALOthman, Z.A. et al. Removal of BrO3 − from drinking water samples using newly developed agricultural waste-based activated carbon and its determination by ultra-performance liquid chromatography-mass spectrometry. Environ Sci Pollut Res 22, 15853–15865 (2015). https://doi.org/10.1007/s11356-015-4786-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4786-y