Abstract
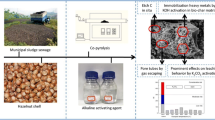
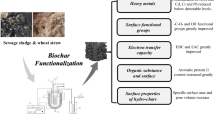
The co-pyrolysis technology was applied to municipal sewage sludge (MSS) and hazelnut shell with alkaline activating agent K2CO3 under N2 atmosphere. The innovative bio-char produced by co-pyrolysis had significant physical and chemical characteristics. The specific surface area reached 1990.23 m2/g, and the iodine absorption number was 1068.22 mg/g after co-pyrolysis at 850 °C. Although hazelnut shell was a kind of solid waste, it also had abundant cellulose resource, which could contribute to porous structure of bio-char during co-pyrolysis with MSS and decrease total heavy metals contents of raw material to increase security of bio-chars. Meanwhile, the residual fractions of heavy metals in bio-char were above 92.95% after co-pyrolysis at 900 °C except Cd to prevent heavy metals digestion, and the bio-char presented significant immobilization behavior from co-pyrolysis technology. Moreover, the yield and the iodine absorption number of bio-chars under different process variables were analyzed, and it was confirmed that appropriate process variables could contribute the yield and the iodine absorption number of bio-char and prevent to etch pore structure excessively to collapse. The changes of surface functional groups and crystallographic structure before and after co-pyrolysis were analyzed by FTIR and XRD, respectively. The hierarchical porous structure of bio-char was presented by SEM and N2 adsorption-desorption isotherm. The Cu(II) adsorption capacity of the bio-char was 42.28 mg/g after 24 h, and surface functional groups acted as active binding sites for Cu(II) adsorption. Langmuir model and pseudo-second-order model can describe process of Cu(II) adsorption well.







Similar content being viewed by others
References
Agrafioti E, Bouras G, Kalderis D, Diamadopoulos E (2013) Biochar production by sewage sludge pyrolysis. J Anal Appl Pyrol 101:72–78
Baek J, Lee HM, Roh JS, Lee HS, Kang HS, Kim BJ (2016) Studies on preparation and applications of polymeric precursor-based activated hard carbons: I. Activation mechanism and microstructure analyses. Micropor Mesopor Mat 219:258–264
Bakisgan C, Dumanli AG, Yürüm Y (2009) Trace elements in Turkish biomass fuels: ashes of wheat straw, olive bagasse and hazelnut shell. Fuel 88(10):1842–1851
Bogusz A, Nowak K, Stefaniuk M, Dobrowolski R, Oleszczuk P (2017) Synthesis of biochar from residues after biogas production with respect to cadmium and nickel removal from wastewater. J Environ Manag 201:268–276
Bondarczuk K, Markowicz A, Piotrowska-Seget Z (2016) The urgent need for risk assessment on the antibiotic resistance spread via sewage sludge land application. Environ Int 87:49–55
Cao YC, Pawłowski A (2012) Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: brief overview and energy efficiency assessment. Renew Sust Energ Rev 16(3):1657–1665
Cao JS, Lin JX, Fang F, Zhang MT, Hu ZR (2014) A new absorbent by modifying walnut shell for the removal of anionic dye: kinetic and thermodynamic studies. Bioresour Technol 163:199–205
Chen D, Yin L, Wang H, He P (2014) Pyrolysis technologies for municipal solid waste: a review. Waste Manag 34(12):2466–2486
Chen FF, Hu YY, Dou XM, Chen DZ, Dai XH (2015) Chemical forms of heavy metals in pyrolytic char of heavy metal-implanted sewage sludge and their impacts on leaching behaviors. J Anal Appl Pyrolysis 116:152–160
Fang W, Wei YH, Liu JG (2016) Comparative characterization of sewage sludge compost and soil: heavy metal leaching characteristics. J Hazard Mater 310:1–10
Fernando NL, Fedorak PM (2005) Changes at an activated sludge sewage treatment plant alter the numbers of airborne aerobic microorganisms. Water Res 39:4597–4608
Fytili D, Zabaniotou A (2008) Utilization of sewage sludge in EU application of old and new methods—a review. Renew Sust Energ Rev 12(1):116–140
Hoşgün EZ, Berikten D, Kıvanç M, Bozan B (2017) Ethanol production from hazelnut shells through enzymatic saccharification and fermentation by low-temperature alkali pretreatment. Fuel 196:280–287
Huang HJ, Yuan XZ (2016) The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Bioresour Technol 200:991–998
Hunsom M, Autthanit C (2013) Adsorptive purification of crude glycerol by sewage sludge-derived activated carbon prepared by chemical activation with H3PO4, K2CO3 and KOH. Chem Eng J 229:334–343
Jin JW, Li YN, Zhang JY, Wu SC, Cao YC, Liang P, Zhang J, Wong MH, Wang MY, Shan SD, Christie P (2016) Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J Hazard Mater 320:417–426
Jin JW, Wang MY, Cao YC, Wu SC, Liang P, Li YN, Zhang JY, Zhang J, Wong MH, Shan SD, Christie P (2017) Cumulative effects of bamboo sawdust addition on pyrolysis of sewage sludge: biochar properties and environmental risk from metals. Bioresour Technol 228:218–226
Katherine FM, Christine MD (2003) Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal Chim Acta 478(1):111–118
Li M, Liu Q, Guo LJ, Zhang YP, Lou ZJ, Wang Y, Qian GR (2013) Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresour Technol 141:83–88
Martin MJ, Artola A, Balaguer MD, Rigola M (2003) Activated carbons developed from surplus sewage sludge for the removal of dyes from dilute aqueous solutions. Chem Eng J 94(3):231–239
Okman I, Karagöz S, Tay T, Erdem M (2014) Activated carbons from grape seeds by chemical activation with potassium carbonate and potassium hydroxide. Appl Surf Sci 293:138–142
Pellera FM, Giannis A, Kalderis D, Anastasiadou K, Stegmann R, Wang JY, Gidarakos E (2012) Adsorption of Cu(II) ions from aqueous solutions on biochars prepared from agricultural by-products. J Environ Manag 96(1):35–42
Rauret G, López-Sánchez JF, Sahuquillo A, Barahona E, Lachica M, Ure AM, Davidson CM, Gomez A, Lück D, Bacon J, Yli-Halla M, Muntau H, Quevauviller P (2000) Application of a modified BCR sequential extraction (three-step)procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM 483),complemented by a three-year stability study of acetic acid and EDTA extractable metal content. J Environ Monitor 2(3):228–233
Shao JG, Yuan XZ, Leng LJ, Huang HJ, Jiang LB, Wang H, Chen XH, Zeng GM (2015) The comparison of the migration and transformation behavior of heavy metals during pyrolysis and liquefaction of municipal sewage sludge, paper mill sludge, and slaughterhouse sludge. Bioresour Technol 198:16–22
Shi W, Liu C, Ding D, Lei Z, Yang Y, Feng C, Zhang Z (2013) Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour Technol 137:18–24
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity. Pure Appl Chem 57(4):603–619
Song X, Li K, Ning P, Wang C, Sun X, Tang LH, Ruan HT, Han S (2017) Surface characterization studies of walnut-shell biochar catalysts for simultaneously removing of organic sulfur from yellow phosphorus tail gas. Appl Surf Sci 425:130–140
Tran HN, You SJ, Chao HP (2017) Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J Environ Manag 188:322–336
Wang P, Tang L, Wei X, Zeng GM, Zhou YY, Deng YC, Wang JJ, Xie ZH, Fang W (2017) Synthesis and application of iron and zinc doped biochar for removal of p-nitrophenol in wastewater and assessment of the influence of co-existed Pb(II). Appl Surf Sci 392:391–401
Wei LL, Zhao QL, Hu K, Lee DJ, Xie CM, Jiang JQ (2011) Extracellular biological organic matters in sewage sludge during mesophilic digestion at reduced hydraulic retention time. Water Res 45:1472–1480
Xu XY, Zhao B, Sun ML, Chen X, Zhang MC, Li HB, Xu SC (2017) Co-pyrolysis characteristics of municipal sewage sludge and hazelnut shell by TG-DTG-MS and residue analysis. Waste Manag 62:91–100
Xue GH, Gao ML, Gu Z, Luo ZX, Hu ZC (2013) The removal of p-nitrophenol from aqueous solutions by adsorption using gemini surfactants modified montmorillonites. Chem Eng J 218:223–231
Yuan X, Huang H, Zeng G, Li H, Wang J, Zhou C, Zhu H, Pei X, Liu Z, Liu Z (2011) Total concentrations and chemical speciation of heavy metals in liquefaction residues of sewage sludge. Bioresour Technol 102(5):4104–4110
Yuan H, Lu T, Zhao D, Huang H, Noriyuki K, Chen Y (2013) Influence of temperature on product distribution and biochar properties by municipal sludge pyrolysis. J Mater Cycles Waste Manage 15(3):357–361
Zhang FS, Nriagu JO, Itoh H (2005) Mercury removal from water using activated carbons derived from organic sewage sludge. Water Res 39(2):389–395
Zhao B, Xu XY, Xu SC, Chen X, Li HB, Zeng FQ (2017) Surface characteristics and potential ecological risk evaluation of heavy metals in the bio-char produced by co-pyrolysis from municipal sewage sludge and hazelnut shell with zinc chloride. Bioresour Technol 243:375–383
Zhao B, Xu XY, Li HB, Chen X, Zeng FQ (2018) Kinetics evaluation and thermal decomposition characteristics of co-pyrolysis of municipal sewage sludge and hazelnut shell. Bioresour Technol 247:21–29
Funding
This research work was supported by the National Water Pollution Control and Management Technology Major Projects (2014ZX07201-009-04), the Liaoning Province Natural Science Foundation (2014020036), and the Fundamental Research Funds for the Central Universities (N160106005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Zhao, B., Xu, X., Zeng, F. et al. The hierarchical porous structure bio-char assessments produced by co-pyrolysis of municipal sewage sludge and hazelnut shell and Cu(II) adsorption kinetics. Environ Sci Pollut Res 25, 19423–19435 (2018). https://doi.org/10.1007/s11356-018-2079-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2079-y