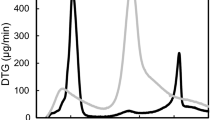
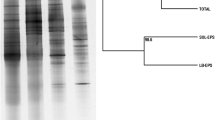
Abstract
In this study, two types of extracellular polysaccharides (EPS), namely, mixed EPS (MX-EPS) and tightly bound EPS (TB-EPS), were extracted from cyanobacterial blooms using different methods. To evaluate their compositional differences, elemental composition, FTIR, and TG/DTA profile were measured for both EPS samples. Following that, unicellular Microcystis aeruginosa was cultured in a medium containing EPS, Ca2+ ion, and Mg2+ ion, and the effect of each type of EPS on the colony formation of M. aeruginosa was examined. Results showed that TB-EPS had more carboxy groups than MX-EPS, and that the TB-EPS medium contained Ca2+ and Mg2+ ions. These cations were not detected in the MX-EPS medium. During the colony formation experiment, colonies were observed when Ca2+ and Mg2+ ions were present at 250 mg/L concentration each. In addition, colony density increased when TB-EPS was added, compared to that of MX-EPS. Colonies were also observed in the medium containing only TB-EPS (100 mg/L), indicating that M. aeruginosa can form colonies using Ca2+ ion present in TB-EPS. During the MX-EPS extraction, Ca2+ ion chelated with EDTA was removed during ethanol precipitation. Therefore, the extraction protocol followed for TB-EPS was better than that of MX-EPS for maintaining Ca2+ ions, and thereby maintaining an EPS composition that enables for colony formation.






Similar content being viewed by others
References
Amemiya Y, Nakayama O (1984) The chemical composition and metal adsorption capacity of the sheath materials isolated from Microcystis, Cyanobacteria. Jpn J Limnol 45:187–193 (in Japanese)
Bi X, Dai W, Zhang S, Xing K, Zhang X (2015) Accumulation and distribution characteristics of heavy metals in different size Microcystis colonies from natural waters. Fresenius Environ Bull 24:773–779
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fattom A, Shilo M (1984) Hydrophobicity as an adhesion mechanism of benthic cyanobacteria. Appl Environ Microbiol 47:135–143
Figueiredo JL, Pereira MFR, Freitas MMA, Orfao JJM (1999) Modification of the surface chemistry of activated carbons. Carbon 37:1379–1389
Forni C, Telo’ FR, Caiola MG (1997) Comparative analysis of the polysaccharides produced by different species of Microcystis (Chroococcales, Cyanophyta). Phycologia 36:181–185
Graham JL, Loftin KA, Meyer MT, Ziegler AC (2010) Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States. Environ Sci Technol 44:7361–7368
Guillard RR, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide C. J Phycol 8:10–14
Hadjoudja S, Deluchat V, Baudu M (2010) Cell surface characterisation of Microcystis aeruginosa and Chlorella vulgaris. J Colloid Interface Sci 342:293–299
Hassler CS, Alasonati E, Nichols CM, Slaveykova VI (2011) Exopolysaccharides produced by bacteria isolated from the pelagic Southern Ocean - role in Fe binding, chemical reactivity, and bioavailability. Mar Chem 123:88–98
Hou J, Yang Y, Wang P, Wang C, Miao L, Wang X, Lv B, You G, Liu Z (2017) Effects of CeO2, CuO, and ZnO nanoparticles on physiological features of Microcystis aeruginosa and the production and composition of extracellular polymeric substances. Environ Sci Pollut Res 24:226–235
Jia YF, Thomas KM (2000) Adsorption of cadmium ions on oxygen surface sites in activated carbon. Langmuir 16:1114–1122
Li P, Cai Y, Shi L, Geng L, Xing P, Yu Y, Kong F, Wang Y (2009) Microbial degradation and preliminary chemical characterization of Microcystis exopolysaccharides from a cyanobacterial water bloom of Lake Taihu. Int Rev Hydrobiol 94:645–655
Li M, Zhu W, Gao L, Lu L (2013) Changes in extracellular polysaccharide content and morphology of Microcystis aeruginosa at different specific growth rates. J Appl Phycol 25:1023–1030
Li M, Zhu W, Sun Q (2014) Solubilisation of mucilage induces changes in Microcystis colonial morphology. N Z J Mar Freshw Res 48:38–47
Liu Y, Wang W, Geng L, Chen Y, Yang Z (2011) Polysaccharide content and morphology of Microcystis aeruginosa in response to changes in metabolic carbon flux. Fresenius Environ Bull 20:1046–1050
Medrano EA, Uittenbogaard RE, Pires LD, Van De Wiel BJH, Clercx HJH (2013) Coupling hydrodynamics and buoyancy regulation in Microcystis aeruginosa for its vertical distribution in lakes. Ecol Model 248:41–56
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nishikawa S, Kuriyama M (1974) Nucleic acid as a component of mucilage in activated sludge. J Ferment Technol 52:335–338 (in Japanese)
Omori K, Sato M, Amano Y, Machida M (2018) Induction of colony formation of Microcystis aeruginosa by controlling extracellular polysaccharides and metal cation concentrations. J Chem Eng Jpn 51:289–297
Otsuka S, Suda S, Li R, Matsumoto S, Watanabe MM (2000) Morphological variability of colonies of Microcystis morphospecies in culture. J Gen Appl Microbiol 46:39–50
Paerl HW, Huisman J (2008) Blooms like it hot. Science 320:57–58
Qu F, Liang H, He J, Ma J, Wang Z, Yu H, Li G (2012) Characterization of dissolved extracellular organic matter (dEOM) and bound extracellular organic matter (bEOM) of Microcystis aeruginosa and their impacts on UF membrane fouling. Water Res 46:2881–2890
Qu F, Du X, Liu B, He J, Ren N, Li G, Liang H (2015) Control of ultrafiltration membrane fouling caused by Microcystis cells with permanganate preoxidation: significance of in situ formed manganese dioxide. Chem Eng J 279:56–65
Reynolds CS (1987) Cyanobacterial water blooms. In: Callow J (ed) Advances in botanical research. Academic Press, London, pp 67–143
Reynolds CS (2007) Variability in the provision and function of mucilage in phytoplankton: facultative responses to the environment. Hydrobiologia 578:37–45
Sato M, Amano Y, Machida M, Imazeki F (2017a) Colony formation of highly dispersed Microcystis aeruginosa by controlling extracellular polysaccharides and calcium ion concentrations in aquatic solution. Limnology 18:111–119
Sato M, Omori K, Datta T, Amano Y, Machida M (2017b) Influence of extracellular polysaccharides and calcium ion on colony formation of unicellular Microcystis aeruginosa. Environ Eng Sci 34:149–157
Sheng GP, Yu HQ, Yu Z (2005) Extraction of extracellular polymeric substances from the photosynthetic bacterium Rhodopseudomonas acidophila. Appl Microbiol Biotechnol 67:125–130
Sheng GP, Yu HQ, Li XY (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol Adv 28:882–894
Shi JQ, Wu ZX, Song LR (2013) Physiological and molecular responses to calcium supplementation in Microcystis aeruginosa (Cyanobacteria). N Z J Mar Freshw Res 47:51–61
Toyoshima C (2008) Structural aspects of ion pumping by Ca2+ -ATPase of sarcoplasmic reticulum. Arch Biochem Biophys 476:3–11
Walsby AE, Hayes PK, Boje R (1995) The gas vesicles, buoyancy and vertical distribution of cyanobacteria in the Baltic Sea. Eur J Phycol 30:87–94
Wang YW, Zhao J, Li JH, Li SS, Zhang LH, Wu M (2011) Effects of calcium levels on colonial aggregation and buoyancy of Microcystis aeruginosa. Curr Microbiol 62:679–683
Wang LL, Wang LF, Ren XM, Ye XD, Li WW, Yuan SJ, Sun M, Sheng GP, Yu HQ, Wang XK (2012) pH dependence of structure and surface properties of microbial EPS. Environ Sci Technol 46:737–744
Xiao M, Willis A, Burford MA, Li M (2017) Review: a meta-analysis comparing cell-division and cell-adhesion in Microcystis colony formation. Harmful Algae 67:85–91
Xiao M, Li M, Reynolds CS (2018) Colony formation in the cyanobacterium Microcystis. Biol Rev 93:1399–1420
Xu H, Yu G, Jiang H (2013) Investigation on extracellular polymeric substances from mucilaginous cyanobacterial blooms in eutrophic freshwater lakes. Chemosphere 93:75–81
Yamamoto Y, Nakahara H (2009) Seasonal variations in the morphology of bloom-forming cyanobacteria in a eutrophic pond. Limnology 10:185–193
Yang Z, Kong F (2013) Abiotic factors in colony formation: effects of nutrition and light on extracellular polysaccharide production and cell aggregates of Microcystis aeruginosa. Chin J Oceanol Limnol 31:796–802
Yang Z, Kong F, Shi X, Zhang M, Xing P, Cao H (2008) Changes in the morphology and polysaccharide content of Microcystis aeruginosa (Cyanobacteria) during flagellate grazing. J Phycol 44:716–720
Zhang K, Lin TF, Zhang T, Li C, Gao N (2013) Characterization of typical taste and odor compounds formed by Microcystis aeruginosa. J Environ Sci 25:1539–1548
Zhao L, Lu L, Li M, Xu Z, Zhu W (2011) Effects of Ca and Mg levels on colony formation and EPS content of cultured M. aeruginosa. Procedia Environ Sci 10:1452–1458
Zhu W, Dai X, Li M (2014a) Relationship between extracellular polysaccharide (EPS) content and colony size of Microcystis is colonial morphology dependent. Biochem Syst Ecol 55:346–350
Zhu W, Li M, Luo Y, Dai X, Guo L, Xiao M, Huang J, Tan X (2014b) Vertical distribution of Microcystis colony size in Lake Taihu: its role in algal blooms. J Gt Lakes Res 40:949–955
Zou H, Pan G, Chen H, Yuan X (2006) Removal of cyanobacterial blooms in Taihu Lake using local soils II. Effective removal of Microcystis aeruginosa using local soils and sediments modified by chitosan. Environ Pollut 141:201–205
Acknowledgments
The authors would like to extend deep gratitude to Prof. Dr. Fumio Imazeki, Safety and Health Organization, Chiba University, for the encouragement of this study.
Funding
This work was partially supported by JFE 21st Century Foundation and by the Japan Society for the Promotion of Science (JSPS) under a Grant-in-Aid for Scientific Research (C) (No. 18 K04404).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vitor Manuel Oliveira Vasconcelos
Rights and permissions
About this article
Cite this article
Omori, K., Datta, T., Amano, Y. et al. Effects of different types of extracellular polysaccharides isolated from cyanobacterial blooms on the colony formation of unicellular Microcystis aeruginosa. Environ Sci Pollut Res 26, 3741–3750 (2019). https://doi.org/10.1007/s11356-018-3892-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3892-z