Abstract
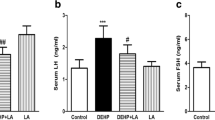
Phthalates, plasticizing chemicals, are top-rated environmental contaminants. Diethyl phthalate (DEP), a chief member of this family, was declared a potent endocrine disruptor and carcinogen in animals and humans. The current study was designed to explore the probable reproductive damage induced by DEP and the therapeutic efficacy of raw honey in male albino mice. Four-week-old 50 male mice were randomized equally in five groups, as control (C) received 0.1 ml distilled water; vehicle control (VC) received corn oil (0.1 ml/mouse); DEP (3mg/g/BW) dissolved in corn oil; honey control (HC) administered with honey (0.2 mg/g/day); and phthalate plus honey (P+H) administered with DEP and honey (3mg and 0.2 mg/g/BW/day respectively). Mice were treated through oral gavage for 54 days routinely, acclimatized for 6 days, and dissected. In the first instance, the antioxidant potential and total phenolic contents (TPC) of honey were analyzed through ferric reducing antioxidant power (FRAP) assay and Folin-Ciocalteu assay to confirm the antioxidant capacity of honey. The morphological, morphometric, histological, micrometric, sperm count, and hormonal analyses, and antioxidant capacity test in tissue homogenates were conducted by using tissues (testis, epididymis) and blood samples of mice. Mice exposed to DEP have a significant increase in body weight, LH level, and seminiferous tubule lumen diameter and decrease in the gonado-somatic index, testosterone level, sperm count, and seminiferous tubule diameter. Additionally, histopathology of testes showed interstitial space dilations, exfoliations, Leydig cell atrophy, germ cell degenerations, and spermatid retention in DEP-exposed testes sections. However, concomitant use of honey and DEP had shown a significant improvement in histopathological lesions, steroid hormone levels, and healthy sperm count. By these results, it is concluded that honey possessed antioxidant potential that can efficiently protect DEP-induced anomalies in male mice.





Similar content being viewed by others
References
Adebolu TT (2005) Effect of natural honey on local isolates of diarrhea causing bacteria in Southwestern Nigeria. Afr J Biotechnol 4:1172–1174
Ali S, Hussain S, Khan R, Mumtaz S, Ashraf N, Andleeb S, Shakir HA, Tahir HM, Khan MKA, Ulhaq M (2019) Renal toxicity of heavy metals (cadmium and mercury) and their amelioration with ascorbic acid in rabbits. Environ Sci Pollut Res Int 26(4):3909–3920
Ali S, Awan Z, Mumtaz S, Shakir HA, Ahmad F, Tahir HM, Ulhaq M (2020a) Cardiac toxicity of heavy metals (cadmium and mercury) and pharmacological intervention by vitamin C in rabbits. Environ Sci Pollut Res Int 27(23):29266–29279
Ali S, Bashir S, Mumtaz S, Shakir HA, Ara C, Ahmad F, Tahir HM, Faheem M, Irfan M, Masih M, Ulhaq M, Andleeb A (2020b) Evaluation of cadmium chloride-induced toxicity in chicks via hematological, biochemical parameters and cadmium level in tissues. Environ Sci Pollut. https://doi.org/10.1007/s12011-020-02453-9
Ali S, Ejaz M, Dar KK, Nasreen N, Ashraf N, Gillani SF, Shafi N, Safeer S, Khan MA, Andleeb S, Mughal TA (2020c) Evaluation of chemopreventive and chemotherapeutic effect of Artemisia vulgaris against diethylnitrosamine induced hepatocellular carcinogenesis in Balb C mice. Braz J Biol 80(3):489–496
Ali S, Bashir S, Mumtaz S et al (2020d) Evaluation of Cadmium Chloride-Induced Toxicity in Chicks Via Hematological, Biochemical Parameters, and Cadmium Level in Tissues. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02453-9
Andjelkovic M, Djordjevic AB, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D et al (2019) Toxic E_ect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16:274
Ara C, Asmatullah BN, Ali S, Batool F, Shakir HA, Arshad A (2020) Abnormal steroidogenesis, oxidative stress and reprotoxicity following prepubertal exposure to butyl paraben in mice and protective effect of Curcuma longa. Environ Sci Pol 28:6111–6121. https://doi.org/10.1007/s11356-020-10819-8
Aswin PS, Neelusree P (2019) To study the antimicrobial properties of honey on common microorganisms. Int J Curr Microbiol App Sci 8(9):114–121
Bancroft JD, Layton C (2013) The hematoxylin and eosin. In: Suvarna SK, Layton C, Bancroft JD (eds) Theory practice of histological techniques, 7th edn. Ch. 10 and 11. Churchill Livingstone of El Sevier, Philadelphia, pp 179–220
Banihani SA. (2019) Mechanisms of honey on testosterone levels, Heliyon. 2019. 5,(7):e02029,
Barakat R, Lin P-C, Park CJ, Zeineldin M, Zhou S, Rattan S, Brehm E, Flaws JA, Ko CMJ (2020) Germline-dependent transmission of male reproductive traits induced by an endocrine disruptor, di-2-ethylhexyl phthalate, in future generations. Sci Rep 10:5705
Blassa M, Candracci M, Accorsi A, Piacentini MP, Albertini MC, Piatti E (2006) Raw millefiori honey is packed full of antioxidants. Food Chem 97:217–222
Buha A, Antonijevic B, Bulat Z, Jacevic V, Milovanović V, Matović V (2013) The impact of prolonged cadmium exposure and co-exposure with polychlorinated biphenyls on thyroid function in rats. Toxicol Lett 221:83–90
Buha A, Matovic V, Antonijevic B, Bulat Z, Čurcíć M, Renieri EA, Tsatsakis AM, Schweitzer A, Wallace D (2018) Overview of cadmium thyroid disrupting effects and mechanisms. Int J Mol Sci:19,1501
Dar KK, Ali S, Ejaz M, Nasreen S, Ashraf N, Gillani SF, Shafi N, Safeer S, Khan MA, Andleeb S, Mughal TA (2019) In vivo induction of hepatocellular carcinoma by diethylnitrosoamine and pharmacological intervention in balb c mice using Bergeniaciliata extracts. Braz J Biol 79(4):629–638
Djordjevic AB, Antonijevic E, Curcic M, Milovanovic V, Antonijevic B (2019) Endocrine disrupting mechanisms of polychlorinated biphenyls. Curr Opin Toxicol 19:42–49
Docea AO, Gofita E, Goumenou M, Calina D, Rogoveanu O, Varut M, Olaru C, Kerasioti E, Fountoucidou P, Taitzoglou I, Zlatian O, Rakitskii VN, Hernandez AF, Kouretas D, Tsatsakis A (2018) Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem Toxicol 115:470–481
Docea AO, Goumenou M, Calina D, Arsene AL, Dragoi CM, Gofita E, Pisoschi CG, Zlatian O, Stivaktakis PD, Nikolouzakis TK, Kalogeraki A, Izotov BN, Galateanu B, Hudita A, Calabrese EJ, Tsatsakis A (2019) Adverse and hormetic effects in rats exposed for 12 months to low dose mixture of 13 chemicals: RLRS part III. Toxicol Lett 310:70–91
Gani KM, Kazmi AA (2020) Ecotoxicological risk evaluation and regulatory compliance of endocrine disruptor phthalates in a sustainable wastewater treatment scheme. Environ Sci Pollut Res 27(8):7785–7794
Gopalakrishnan K, Aushev VN, Manservisi F, Falcioni L, Panzacchi S, Belpoggi F, Parada H, Garbowski G, Hibshoosh H, Santella RM, Gammon MD (2020) Gene expression profiles for low-dose exposure to diethyl phthalate in rodents and humans: a translational study with implications for breast carcinogenesis. Sci Rep 10(1):1–12
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT (2015) EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev 36:1–150
Ha M, Guan X, Wei L, Li P, Yang M, Liu C (2016) Di-(2-ethylhexyl) phthalate inhibits testosterone level through disturbed hypothalamic–pituitary–testis axis and ERK-mediated 5α-Reductase 2. Sci Total Environ 563:566–575
Harley KG, Berger K, Rauch S, Kogut K, Henn BC, Calafat AM, Huen K, Eskenazi B, Holland N (2017) Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr Res 82(3):405–415
Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, Calafat AM (2007) DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod 22(3):688–695
Hernandez AF, Buha A, Constantin C, Wallace DR, Sarigiannis D, Neagu M, Antonijevic B, Hayes AW, Wilks MF, Tsatsakis A (2019) Critical assessment and integration of separate lines of evidence for risk assessment of chemical mixtures. Arch Toxicol 93:2741–2757
Hossen MS, Ali MY, Jahurul MHA, Mohamed M, Abdel-Daim, Gan SH, Khalil MI (2017) Beneficial roles of honey polyphenols against some human degenerative diseases: a review. Pharmacol Rep 69(6):1194–1205
Hurst CH, Waxman DJ (2003) Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol Sci 74(2):297–308
Hussain S, Ali S, Mumtaz S, Shakir HA, Ahmad F, Tahir HM, Ulhaq M (2020) Dose and duration-dependent toxicological evaluation of lead acetate in chicks. Environ Sci Pollut Res 27(13):15149–15164
Ibrahim A, Abd Eldaim MA, Abdel-Daim MM (2016) Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology 68(4):1039–1048. https://doi.org/10.1007/s10616-015-9860-2
James AW, Beverly BEJ, Keshava N, Mudipalli A, Arzuaga X, Cai C, Hotchkiss AK, Makris SL, Yost EE (2020) Hazards of diethyl phthalate (DEP) exposure: a systematic review of animal toxicology studies. Environ Int 145:105848
Jeong HJ, Shin YG, Kim IH, Pezzuto JM (1999) Inhibition of aromatase activity by flavonoids. Arch Pharm Res 22(3):309–312
Jeong SH, Jang JH, Cho HY, Lee YB (2020) Risk assessment for humans using physiologically based pharmacokinetic model of diethyl phthalate and its major metabolite, monoethyl phthalate. Arch Toxicol:1–24
Kaabi AM, Barakat IAH, Alajmi RA, Abdel-Daim MM (2020) Use of black seed (Nigella sativa) honey bee to improve sheep oocyte maturation medium. Environ Sci Pollut Res 27:33872–33881. https://doi.org/10.1007/s11356-020-09504-7
Kadiri, H., 2018. The ameliorating effects of honey on some biochemical parameters on rats exposed to cyanide. Biokemistri, 30(1).
Katalinica V, Modun D, Music I, Boban M (2005) Gender differences in antioxidant capacity of rat tissues determined by 2, 2′-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 140(1):47–52
Khan R, Ali S, Mumtaz M, Andleeb S, Ulhaq M, Tahir HM, MKA K, Khan MA, Shakir HA (2019) Toxicological effects of heavy metals (cadmium and mercury) on blood and thyroid gland and pharmacological intervention by vitamin c in rabbits. Environ Sci Pollut Res 26(16):16727–16741
Kirkman-Brown J, Björndahl L (2009) Evaluation of a disposable plastic Neubauer counting chamber for semen analysis. Fertil Steril 91(2):627–631
Kolawole TA, Oyeyemi WA, Adigwe C, Leko B, Udeh C, Dapper DV (2015) Honey attenuates the detrimental effects of nicotine on testicular functions in nicotine treated wistar rats. Nigerian Journal of Physiological Sciences 30(1-2):10–16
Li K, Liszka M, Zhou C, Brehm E, Flaws JA, Nowak RA (2020a) Prenatal exposure to a phthalate mixture leads to multigenerational and transgenerational effects on uterine morphology and function in mice. Reprod Toxicol 93:178–190
Li X, Wan J, Wang Y, Yan Z, Chi H, Ding S (2020b) Mechanism of accurate recognition and catalysis of diethyl phthalate (DEP) in wastewater by novel MIL100 molecularly imprinted materials. Appl Catal B Environ:118591
Meo SA, Al-Asiri SA, Mahesar AL, Ansari MJ (2017) Role of honey in modern medicine. Saudi J Biol Sci 24(5):975–978
Mohamed M, Sulaiman SA, Jaafar H, Sirajudeen KN (2011 Sep) Antioxidant protective effect of honey in cigarette smoke-induced testicular damage in rats. Int J Mol Sci 12(9):5508–5521
Mondal S, Ghosh S, Bhattacharya S, Mukherjee S (2019) Chronic dietary administration of lower levels of diethyl phthalate induces murine testicular germ cell inflammation and sperm pathologies: Involvement of oxidative stress. Chemosphere. 229:443–451
Montoto LG, Arregui L, Sánchez NM, Gomendio M, Roldan ER (2012) Postnatal testicular development in mouse species with different levels of sperm competition. Reproduction 143(3):333
Mosavat M, Mohamed M, Ooi FK, Mirsanjari M, Zin AAM, Romli AC (2019) Histological changes of female reproductive organs subjected to different jumping exercise intensities and honey supplementation in rats. PeerJ 7:e7646
Mughal TA, Saleem MZ, Ali S, Anwar KK, Bashir MM, Babar M, Khan MA (2019) Evaluation of hepatotoxicity of carbon tetrachloride and pharmacological intervention by vitamin E in balb c mice. Pakistan Journal of Zoology 51(2):755–761
Mughal TA, Ali S, Hassan A, Saleem MZ, Mumtaz S, Mumtaz S (2020) Carbon Tetrachloride-Induced Hepatocellular Damage in Balb C Mice and Pharmacological Intervention by Extract of Daucus Carota. RADS J Pharm Pharm Sci 8(4):1–9
Mumtaz S, Ali S, Khan R, Andleeb S, Ulhaq M, Khan MA, Shakir HA (2019) The protective role of ascorbic acid in the hepatotoxicity of cadmium and mercury in rabbits. Environ SciPollut Res Int 26(14):14087–14096
Murosak S, Muroyama K, Yamamoto Y, Liu T, Yoshikai Y (2002) Nigerooligosaccharides augments natural killer activity of hepatic mononuclear cells in mice. Int Immunopharmacol 2:151–159
Mustafa S, Wei Q, Ennab W, Lv Z, Nazar K, Siyal FA, Rodeni S, Ngekure M, Kavita X, Shi F (2019) Resveratrol ameliorates Testicular histopathology of mice exposed to restraint stress. Animals 9:743
Naeem S, Ashraf M, Babar ME, Zahoor S, Ali S (2021) The effects of some heavy metals on some fish species. Environ Sci Pollut Res 28(20):25566–25578
Nasrolahi O, Khaneshi F, Rahmani F, Razi M (2013) Honey and metformin ameliorated diabetes-induced damages in testes of rat; correlation with hormonal changes. Iranian journal of reproductive medicine 11(12):1013
Nemoseck TM, Carmody EG, Furchner-Evanson A, Gleason M, Li A, Potter H, Rezende LM, Lane KJ, Kern M (2011) Honey promotes lower weight gain, adiposity, and triglycerides than sucrose in rats. Nutr Res 31(1):55–60
Ozcan-Sezer S, Ince E, Akdemir A, Ceylan ÖÖ, Suzen S, Gurer-Orhan H (2019) Aromatase inhibition by 2-methyl indole hydrazone derivatives evaluated via molecular docking and in vitro activity studies. Xenobiotica. 49(5):549–556
Pipicelli G, Tatti P (2009) Therapeutic properties of honey. Health 1(2):281–283
Radke EG, Braun JM, Nachman RM, Cooper GS (2020) Phthalate exposure and neurodevelopment: a systematic review and meta-analysis of human epidemiological evidence. Environ Int 137:105408
Rahman M, Brazel CS (2004) The plasticizer market: an assessment of traditional plasticizers and research trends to meet new challenges. Prog Polym Sci 29(12):1223–1248
Rodríguez-Carmona Y, Cantoral A, Trejo-Valdivia B et al (2019) Phthalate exposure during pregnancy and long-term weight gain in women. Environ Res 169:26–32
Sedha S, Lee H, Singh S, Kumar S, Jain S, Ahmad A, Bin YA, Jardan SS, Shukla S, Simal-Gandara J, Xiao J, Huh YS, Han Y-K, Bajpai VK (2021) Reproductive toxic potential of phthalate compounds – state of art review. Pharmacol Res 167:105536
Selvaraju K, Vikram P, Soon JM, Krishnan KT, Mohammed A (2019) Melissopalynological, physicochemical and antioxidant properties of honey from West Coast of Malaysia. J Food Sci Technol 56(5):2508–2521
Shaha CM, Pandit RS (2020) Biochemical and molecular changes mediated by plasticizer diethyl phthalate in Chironomus circumdatus (bloodworms). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 228:108650
Tanner EM, Hallerbäck MU, Wikström S, Lindh C, Kiviranta H, Gennings C, Bornehag CG (2020) Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environ Int 134:105185
Tariq M, Tahir HM, Butt SA, Ali S, Ahmad AB, Raza C, Summer M, Hassan A, Nadeem J, (2021) Silk derived formulations for accelerated wound healing in diabetic mice. PeerJ 9:e10232
Terzo S, Mulè F, Amato A (2020) Honey and obesity-related dysfunctions: a summary on health benefits. J Nutr Biochem 82:108401
Tsatsakis AM, Kouretas D, Tzatzarakis MN, Stivaktakis P, Tsarouhas K, Golokhvast KS, Rakitskii,VN, Tutelyan VA, Hernandez AF, Rezaee R et al (2017) Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum Exp Toxicol 36:554–564 [CrossRef]
Vela L, de Lorenzo C, Perez RA (2007) Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J Sci Food Agric 87(6):1069–1075
Wang C, Croll RP (2004) Effects of sex steroids on gonadal development and gender determination in the sea scallop, Placopecten magellanicus. Aquaculture 238(1–4):483–498
Yeung AWK, Tzvetkov NT, El-Tawil OS, Bungǎu SG, Abdel-Daim MM, Atanasov AG (2019) Antioxidants: scientific literature landscape analysis. Oxidative Med Cell Longev 2019:8278454, 11 pages. https://doi.org/10.1155/2019/8278454
Zhou Y, Huang M, Wang X, Gao J, Fang G, Zhou D (2020) Efficient transformation of diethyl phthalate using calcium peroxide activated by pyrite. Chemosphere, p 126662
Acknowledgements
We are thankful to the officials of the the Institute of Zoology, University of the Punjab, Lahore, Pakistan, for providing us research facilities.
Availability of data and materials
Most of the data generated during this study are included in this article. However, raw data sheets and histopathological figures are available from the corresponding as well as co-authors upon reasonable request.
Author information
Authors and Affiliations
Contributions
Conceptualization: Chaman Ara, Asmatullah. Data curation: Chaman Ara, Asmatullah, Faiza Yaseen, Shaukat Ali, Nagina Ramzan, Hafiz Abdullah Shakir, Shagufta Andleeb, Muhammad Khan. Formal analysis: Chaman Ara, Asmatullah, Faiza Yaseen, Shaukat Ali, Nagina Ramzan. Investigation: Chaman Ara, Asmatullah, Shaukat Ali, Hafiz Abdullah Shakir, Muhammad Khan. Methodology: Chaman Ara, Asmatullah, Faiza Yaseen, Shagufta Andleeb, Shaukat Ali, Nagina Ramzan, Hafiz Abdullah Shakir, Muhammad Khan. Histopathology: Faiza Yaseen, Nagina Ramzan, Muhammad Khan. Software: Shaukat Ali, Hafiz Abdullah Shakir. Supervision: Chaman Ara, Asmatullah. Writing original draft: Faiza Yaseen, Nagina Ramzan, Hafiz Abdullah Shakir, Shaukat Ali, Muhammad Khan, Shagufta Andleeb. Review and editing: Chaman Ara, Asmatullah, Shagufta Andleeb, Shaukat Ali, Hafiz Abdullah Shakir.
Corresponding author
Ethics declarations
Ethics approval
All animal trials were executed according to local and worldwide procedures. The nearby way is the Wet op de dierproeven (article 9) of Dutch law (international) and an associated rule planned via the Bureau of Animal Research Licensing, Local University, as detailed in our earlier papers (Ali et al. (Ali et al. 2020), (Ali 2020), (Ali et al. 2020); Hussain et al. (Hussain et al. 2020); Ali et al. (Ali et al. 2020), (Ali 2020), (Ali et al. 2020); Ara et al. (Ara et al. 2020); Ali et al. (Ali et al. 2020), (Ali 2020), (Ali et al. 2020); Khan et al. (Khan et al. 2019); Ali et al. (Ali et al. 2019); Mumtaz et al. (Mumtaz et al. 2019); Mughal et al. (Mughal et al. 2019); Dar et al. (Dar et al. 2019)). The rearing and use of mice were carried out using NIH Publication “Guide for the Care and Use of Laboratory Animals” (NRC 2004) and with the approval vide No. D/681/UZ dated 04-04-2019 by the local bioethical committee of the University on animal experimentation.
Consent to participate
The rearing and use of mice was following NIH Publication “Guide for the Care and Use of Laboratory Animals” (NRC 2004) and with the approval by the local ethical committee of University of the Punjab, Lahore, Pakistan, on animal experimentation (can provide a certificate on demand).
*Statement about this also added in manuscript.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ara, C., Asmatullah, Yaseen, F. et al. Evaluation of sex steroid hormones and reproductive irregularities in diethyl phthalate-exposed premature mice: modulatory effect of raw honey against potential anomalies. Environ Sci Pollut Res 28, 55265–55276 (2021). https://doi.org/10.1007/s11356-021-14774-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14774-w