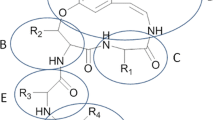
Abstract
The present review describes research on novel natural cyclobutane-containing alkaloids and synthetic compounds isolated from terrestrial and marine species. More than 210 compounds have been confirmed to show antimicrobial, antibacterial, anticancer, and other activities. Structures, origins, biosynthesis, photodimerization, and biological activities of a selection of cyclobutane-containing alkaloids and selected synthethic analogs of natural alkaloids are reviewed.






































































Similar content being viewed by others
References
Dembitsky VM (2005) Astonishing diversity of natural surfactants. 6. Biological active marine and terrestrial alkaloid glycosides. Lipids 40:1081–1105
Dembitsky VM, Gloriozova T, Poroikov VV (2005) Novel antitumor agents: marine sponge alkaloids, their synthetic analogues and derivatives. Mini Rev Med Chem 5:319–336
Dembitsky VM (2002) Bromo- and iodo-containing alkaloids from marine microorganisms and sponges. Russ J Bioorg Chem 28:196–208
Hansen TV, Stenstrom Y (2001) Naturally occurring cyclobutanes. Organic Synthesis: Theor Appl 5:1–38
Avotin’sh FM (1993) Amino acids of cyclobutane series. Usp Khim (Russia) 62:949–958
Ortuno RM, Moglioni AG, Moltrasio GY (2005) Cyclobutane biomolecules: synthetic approaches to amino acids, peptides and nucleosides. Curr Org Chem 9:237–259
Vink AA, Roza L (2001) Biological consequences of cyclobutane pyrimidine dimers. J Photochem Photobiol B 65:101–104
Bellus D, Ernst B (1988) Cyclobutanones and cyclobutenones in nature and in synthesis. Angew Chem Int Ed Engl 27:797–827
Mehta LK, Parrick J (2000) Four-membered ring systems. Prog Heterocyc Chem 12:77–91
Roth HJ (2005) Four-membered rings. Dtsch Apoth Ztg 145:56–62
Rappoport Z, Liebman JF (eds) (2005) The chemistry of cyclobutanes, Parts 1–2. Wiley, Chichester, p 616
Vikram A, Hamzehzarghani H, Kushalappa AC (2005) Volatile metabolites from the headspace of onion bulbs inoculated with postharvest pathogens as a tool for disease discrimination. Can J Plant Pathol 27:194–203
Bell EA, Qureshi MY, Pryce RJ, Janzen DH, Lemke P, Clardy J (1980) 2,4-Methanoproline (2-carboxy-2,4-methanopyrrolidine) and 2,4-methanoglutamic acid (1-amino-1,3-dicarboxycyclobutane) in seeds of Ateleia herbert-smithii Pittier (Leguminosae). J Am Chem Soc 102:1409–1412
Kite GC, Ireland H (2002) Non-protein amino acids of Bocoa (Leguminosae; Papilionoideae). Phytochemistry 59:163–168
Stevens CV, Smagghe G, Rammeloo T, De Kimpe N (2005) Insect repellent/antifeedant activity of 2,4-methanoproline and derivatives against a leaf- and seed-feeding pest insect. J Agric Food Chem 53:1945–1948
Harrison WA, Curcumelli-Rodostamo M, Carson DF, Barclay LRC, MacLean DB (1961) Lycopodium alkaloids. X. The structure of lycopodine. Can J Chem 39:2086–2099
Miles DH, Tunsuwan K, Chittawong V, Kokpol U, Choudhary MI, Clardy J (1993) Boll weevil antifeedants from Arundo donax. Phytochemistry 34:1277–1279
Maki Y (1961) Lycopodium alkaloids. Gifu Yakka Daigaku Kiyo 11:1–8, CA 56:46334
Manske RHF, Marion L (1943) The alkaloids of Lycopodium species. III. Lycopodium annotinum L. Can J Res 21B:92–96
Manske RHF, Marion L (1947) Alkaloids of Lycopodium species. IX. Lycopodium annotinum var. acrifolium Fern. and the structure of annotinine. J Am Chem Soc 69:2126–2169
Wiesner K, Valenta Z, Ayer WA, Bankiewicz C (1956) The structure of annotinine. Chem Ind (London): 1019
Wiesner K, Valenta Z, Ayer WA, Fowler LR, Francis JE (1958) Annotinine-II: the complete structure. Tetrahedron 4:87–104
Ho TL (1969) The stereochemistry of C15 in annotinine. Tetrahedron Lett 10:1307–1308
Wiesner K, Poon L, Jirkovský I, Fishman M (1969) The total synthesis of optically active annotinine. Can J Chem 47:433–444
Wiesner K, Francis JE, Findlay JA, Valenta Z (1961) The configuration of annotinine and some rearrangements. Tetrahedron Lett 2:187–196
Knop O, MacLean DB (1952) Lycopodium alkaloids. I. Physical properties and X-ray crystallographic data of some Lycopodium alkaloids. Can J Chem 30:598–602
Ayer WA, Wichiacz M, Trifonov LS (1999) Annotinine revisited. A new entacyclo [7.3.3.01,13.02,12.05,13] pentadecane ester and other products derived from annotinine. Can J Chem 77:1514–1520
Achmatowicz O, Rodewald W (1955) Lycopodium alkaloids. II. The alkaloids of Lycopodium annotinum. Roczniki Chem (Warsaw) 29:509–530
Perry GS, MacLean DB (1956) Lycopodium alkaloids. III. Functional groups of some minor alkaloids of Lycopodium annotinum. Can J Chem 34:1189–99
Bertho A, Stoll A (1952) Lycopodium alkaloids. I. The alkaloids from Lycopodium annotinum. Chem Ber 85:663–685
Rouffiac R (1961) Alkaloids in Lycopodium phlegmaria. Comp Rend Hebdomad Sean l’Academ Sci (Paris) 253:2612–2613
Achmatowicz O, Rodewald W (1955) The alkaloids of Lycopodium selago. Bull Acad Polonia Sci Class III (Warsaw, Poland) 3:553–555
MacLean DB (1968) Lycopodium alkaloids. In: Manske RHF (eds) Alkaloids, vol. 10. Academic, London, p 306
Ma XQ, Jiang SH, Zhu DY (1998) Alkaloid patterns in Huperzia and some related genera of Lycopodiaceae Sensu Lato occurring in China and their contribution to classification. Biochem Syst Ecol 26:723–728
Leete E (1958) The biogenesis of annotinine. Tetrahedron 3:313–314
Robinson R (1955) The structural relations of Nafurnl products. Clarendon, Oxford, p 72
Koyama K, Morita H, Hirasawa Y, Yoshinaga M, Hoshino T, Obara Y, Nakahata N, Kobayashi J (2005) Lannotinidines A-G, new alkaloids from two species of Lycopodium. Tetrahedron 61:3681–3690
Hartley TG (1986) Three new species of Sarcomelicope (Rutaceae) from New Caledonia (with a new key to the species of the genus). Adansonia 8:183–189
Fokialakis N, Magiatis P, Terzis A, Tillequin F, Skaltsounis A-L (2001) Cyclomegistine, the first alkaloid with the new cyclobuta[b]quinoline ring system from Sarcomelicope megistophylla. Tetrahedron Lett 42:5323–5325
Chosson E, Verite P, Blanckaert A, Seguin E, Litaudon M, Sevenet T (2003) Non polar compounds from the bark of Sarcomelicope follicularis. Biochem Syst Ecol 31:1185–1188
Stobbe H (1919) Constitution of the truxillic acids and of truxone. Ber Dtsch Chem Ges 52B:1021–108
Stoermer R, Foerster G (1919) The truxillic acids and truxones. Ber Dtsch Chem Ges 52B:1255–1272
Stoermer R, Laage E (1921) Truxillic acids. M. Natural and artificial truxinic and truxinic acids. Ber Dtsch Chem Ges 54B:77–85
Stobbe H, Steinberger FK (1922) Light reactions of the cis- and trans-cinnamic acids. Ber Dtsch Chem Ges 55B:2225–2245
Kan RO (1966) Organic photochemistry. McGraw-Hill, New York, p 157
Natarajan A, Ramamurthy V (2005) In: Rappoport Z, Liebman JF (eds) The chemistry of cyclobutanes. Wiley, New York, pp 807–872
Bassani DM (2004) The dimerization of cinnamic acid derivatives. CRC Handbook of organic photochemistry and photobiology, 2nd edn, pp 1–20
Hanley AB, Russell WR, Chesson A (1993) Formation of substituted truxillic and truxinic acids in plant-cell walls—a rationale. Phytochemistry 33:957–960
Krauze-Baranowska M (2002) Truxillic and truxinic acids—occurrence in plant kingdom. Acta Poloniae Pharm Drug Res 59:403–410
Ito Y, Hosomi H, Ohba S (2000) Compelled orientational control of the solid-state photodimerization of trans-cinnamamides: dicarboxylic acid as a non-covalent linker. Tetrahedron 56:6833–6844
Hartmann R, San-Martin A, Munoz O, Breitmaier E (1990) Grahamine, an unusual tropane alkaloid from Schizanthus grahamii. Angew Chem 102:441–443
Rahman AU, Khattak KF, Nighat F, Shabbir M, Hemalal KD, Tillekeratne LM (1998) Dimeric tropane alkaloids from Erythroxylum moonii. Phytochemistry 48:377–383
Anon (1912) Coca leaves from ceylon and federated states. Bull Imperial Instit (London) 10:37–42
Moore JM, Casale JF, Klein RFX, Cooper DA, Lydon J (1994) Determination and in-depth chromatographic analyses of alkaloids in South American and greenhouse-cultivated coca leaves. J Chromatogr 659:163–175
Lurie IS, Moore JM, Kram TC, Cooper DA (1990) Isolation, identification and separation of isomeric truxillines in illicit cocaine. J Chromatogr 504:391–401
Novak M, Salemink CA, Khan I (1984) Biological activity of the alkaloids of Erythroxylum coca and Erythroxylum novogranatense. J Ethnopharmacol 10:261–274
Griffin WJ, Lin GD (2000) Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry 53:623–637
Nehme M, Landa A, Ribas I (1977) Alkaloids of Papilionacea. LXII. Study of alkaloids of Adenocarpus complicatus (L.) Gay, subspecies Aureus (Cav.) Vicioso. An Quim (Brazil) 73:307–308
Ribas I, Talarid P (1950) Adenocarpine and santiaguine, two alkaloids from the broom of Galicia. Mon Farm Terapia (Madrid) 56:377–379
Gonzalez AG, Gonzalez EG, Cartaya LM (1953) The alkaloids of Adenocarpus foliosus. Anal Real Soc Esp Fisica Quim (Madrid) 49B:783–788
Faugeras G (1970) Alkaloids and polyphenols of legumes. XVIII. Adenocarpus mannii alkaloids. Presence of (+)-adenocarpine, isoorensine, and santiaguine in leaves. Plant Med Phytotherap (Paris) 4:9–20
Bernasconi R, Steinegger E (1970) Legume alkaloids. XX. Alkaloids of Adenocarpus mannii, a legume from Kilimanjaro. Pharm Acta Helvet 45:42–51
Ribas I (1960) Alkaloids of species of genus Adenocarpus D.C. Rev Real Acad Ciencia. Exacts: Fisica Natura (Madrid) 54:405–414
Luces J, Dominguez J, Ribas I (1958) Alkaloids of the Papilionaceae. XXXI. The chemistry of orensine and santiaguine. Anal Real Soc Esp: Fisica Quim (Madrid) 54B:215–222
Ribas I (1963) The alkaloids of Adenocarpus species. Abhandl Deut Akad Wiss Geol Biol 4:149–157
Mendez MR, Ribas I (1958) Papilionaceous alkaloids. XXX. Alkaloids of Adenocarpus grandiflorus. Anal Real Soc Esp: Fisica Quim (Madrid) 54B:161–166
Ribas I, Rivera E (1958) Alkaloids of the Papilionaceae. XXI. Isoorensine, a new alkaloid from the Galician hairy cytisus. Anal Real Soc Esp: Fisica Quim (Madrid) 49B:707–710
Gonzalez AG, Gonzalez EG, Cartaya LM (1953) The alkaloids of Adenocarpus foliosus. Publicat Inst Quim “Alonso Barba” (Madrid) 7:232–237
Ribas I, Taladrid P (1950) Adenocarpine and santiaguine, two alkaloids from the broom of Galicia. Anal Real Soc Esp: Fisica Quim (Madrid) 46B:489–500
Fitzgerald JS, Johns SR, Lamberton JA, Redcliffe AH (1972) Alkaloids of Hovea longipes (Leguminosae): the structure of a hypotensive alkaloid. Anal Quim 68:737–742
Lamberton JA, Morton TC, Suares H (1982) Alkaloids of Hovea linearis R.Br. The isolation of Ormosia group alkaloids. Aust J Chem 35:2577–2582
Taylor WC (1991) Bioactive products from plants. Chem Aust 58:56–59
O’Donovan DG, Creedon PB (1974) Biosynthesis of santiaguine in Adenocarpus foliosus. II. J Chem Soc Perkin Trans 1 22:2524–2548
Nehme M, Landa A, Ribas I (1975) Papilionaceae alkaloids. LXI. Structure of meso-santiaguine. Anal Quim 71:627–628
Chi Y, Hashimoto F, Nohara T, Nakamura M, Yoshizawa T, Yamashita M, Marubayashi N (1996) Incarvillea alkaloids and their analgesic and sedative activities. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu (Japan) 38:43–48
Nakamura M, Chi YM, Yan W-M, Yonezawa A, Nakasugi Y, Yoshizawa T, Hashimoto F, Kinjo J, Nohara T, Sakurada S (2001) Structure-antinociceptive activity studies of incarvillateine, a monoterpene alkaloid from Incarvillea sinensis. Planta Med 67:114–117
Chi Y-C, Hashimoto F, Yan W-M, Nohara T (1997) Four monoterpene alkaloid derivatives from Incarvillea sinensis. Phytochemistry 46:763–769
Chi Y-M, Nakamura M, Zhao X-Y, Yoshizawa T, Yan W-M, Hashimoto F, Kinjo J, Nohara T (2005) A monoterpene alkaloid from Incarvillea sinensis. Chem Pharm Bull 53:1178–1179
Beck AB, Goldspink BH, Knox JR (1979) A re-examination of the alkaloids of Lupinus cosentinii (Guss.). J Nat Prod 42:385–398
Mashkovskii MD (1944) Pharmacology of the alkaloid, thesine. Am Rev Soviet Med 2:67–69
Arendaruk AP, Proskurnina NF, Konovalova RA (1960) Alkaloids of Thesium minkwitzianum plant. Zh Obshch Khim (USSR) 30:670–676
Arendaruk AP, Skoldinov AP (1960) Cyclobutanedicarboxylic acids. I. Structure of thesinic acid. Zh Obshch Khim (USSR) 30:484–488
Soltis PA, Soltis DE, Chase MW (1999) Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 402:402–404
Jaramillo MA, Manos PS (2001) Phylogeny and patterns of floral diversity in the genus Piper (Piperaceae). Am J Bot 88:706–716
Wei K, Li W, Koike K, Chen Y, Nikaido T (2005) Nigramides A–S, dimeric amide alkaloids from the roots of Piper nigrum. J Org Chem 70:1164–1176
Fujiwara Y, Naithou K, Miyazaki T, Hashimoto K, Mori K, Yamamoto Y (2001) Two new alkaloids, pipercyclobutanamides A and B, from Piper nigrum. Tetrahedron Lett 42:2497–2499
Lee F-P, Chen Y-C, Chen J-J, Tsai I-L, Chen I-S (2004) Cyclobutanoid amides from Piper arborescens. Helv Chim Acta 87:463–468
Maxwell A, Rampersad D (1991) A new dihydropiplartine and. piplartine dimer from Piper rugosum. J Nat Prod 54:1150–1152
Duh CY, Wu YC, Wang S K (1990) Cytotoxic pyridone alkaloids from the leaves of Piper aborescens. J Nat Prod 53:1575–1577
Hadom H, Jungkunz R (1951) Pepper and cubebs. Pharm Acta Helv 26:25–31
Tsai I-L, Lee F-P, Wu C-C, Duh C-Y, Ishikawa T, Chen J-J, Chen Y-C, Seki H, Chen I-S (2005) New cytotoxic cyclobutanoid amides, a new furanoid lignan and anti-platelet aggregation constituents from Piper arborescens. Planta Med 71:535–542
Dhar KL, Shah S, Prabhakar A, Sharma RL (1995) New pyrrolidinamide dimers from Piper peepuloides. Fitoterapia 66:390–392
Sharma RL, Kumari M, Kumar N, Prabhakar A (1999) New piperidinamide dimers from Piper peepuloides. Fitoterapia 70:144–147
Reddy SM, Surekha M, Reddy VK (1997) Incidence and biology of tremorgenic mycotoxins. Microb Biotechnol 66:252–261
Steyn PS (1992) Nitrogen-containing mycotoxins: tremorgenic mycotoxins. Priklad Biokhim Mikrobiol (USSR/Russia) 28:858–869
Betina V (1989) Structure-activity relationships among mycotoxins. Chem Biol Interact 71:105–146
Cole RJ (1993) Fungal tremorgens. Priklad Biokhim Mikrobiol (Russia) 29:44–50
Steyn PS, Vleggaar R (1985) Tremorgenic mycotoxins. Prog Chem Org Nat Prod 48:1–80
Rundberget T, Skaar I, Flaoyen A (2004) The presence of Penicillium and Penicillium mycotoxins in food wastes. Int J Food Microbiol 90:181–188
Hayashi H (2005) Bioactive alkaloids of fungal origin. Stud Nat Prod Chem 32:549–609
Sonjak S, Frisvad JC, Gunde-Cimerman N (2005) Comparison of secondary metabolite production by Penicillium crustosum strains, isolated from Arctic and other various ecological niches. FEMS Microbiol Ecol 53:51–60
Hayashi H (1998) Fungal metabolites with bioactivity to insects. Recent Res Develop Agric Biol Chem 2:511–525
De Jesus AE, Steyn PS, Van Heerden FR, Vleggaar R, Wessels PL, Hull WE (1983) Tremorgenic mycotoxins from Penicillium crustosum: isolation of penitrems A–F and the structure elucidation and absolute configuration of penitrem A. J Chem Soc Perkin Trans 1 8:1847–1856
De Jesus AE, Steyn PS, Van Heerden FR, Vleggaar R, Wessels PL, Hull WE (1981) Structure and biosynthesis of the penitrems A–F, six novel tremorgenic mycotoxins from Penicillium crustosum. J Chem Soc Chem Commun 6:289–291
Rundberget T, Wilkins AL (2002) Thomitrems A and E, two indole-alkaloid isoprenoids from Penicillium crustosum Thom. Phytochemistry 61:979–985
Rundberget T, Skaar I, O’Brien O, Flaoyen A (2004) Penitrem and thomitrem formation by Penicillium crustosum. Mycopathol 157:349–357
Puschner B (2002) Mycotoxins. The veterinary clinics of North America. Small Anim Pract 32:409–419
Gonzalez MC, Lull C, Moya P, Ayala I, Primo J, Yufera EP (2003) Insecticidal activity of penitrems, including penitrem G, a new member of the family isolated from Penicillium crustosum. J Agric Food Chem 51:2156–2160
Penn J, Biddle JR, Mantle PG, Bilton JN, Sheppard RN (1992) Pennigritrem, a naturally-occurring penitrem A analog with novel cyclization in the diterpenoid moiety. J Chem Soc Perkin Trans 1 1:23–26
Yamaguchi T, Nozawa K, Hosoe T, Nakajima S, Kawai K (1993) Indoloditerpenes related to tremorgenic mycotoxins, penitrems, from Penicillium crustosum. Phytochemistry 32:1177–1181
Laakso JA, Gloer JB, Wicklow DT, Dowd PF (1993) A new penitrem analog with antiinsectan activity from the sclerotia of Aspergillus sulphureus. J Agric Food Chem 41:973–975
Laakso JA, Gloer JB, Wicklow DT, Dowd PF (1992) Sulpinines A–C and secopenitrem B: new antiinsectan metabolites from the sclerotia of Aspergillus sulphureus. J Org Chem 57:2066–2071
Naik JT, Mantle PG, Sheppard RN, Waight ES (1995) Penitremones A–C, Penicillium metabolites containing an oxidized penitrem carbon skeleton giving insight into structure—tremorgenic relationships. J Chem Soc Perkin Trans 1(9):1121–1125
Wilson BJ, Hoekman T, Dettbarn WD (1972) Effects of a fungus tremorgenic toxin (penitrem A) on transmission in rat phrenic nerve-diaphragm preparations. Brain Res 40:540–544
Cysewski SJ, Baetz AL, Pier AC (1975) Penitrem A intoxication of calves: blood chemical and pathologic changes. Am J Veter Res 36:53–58
Malaiyandi M, Vesonder RF, Ciegler A (1976) Large scale production, purification and a study of some spectral properties of Penitrem A. J Environ Sci Health 11:139–164
Hayes AW, Presley DB, Neville JA (1976) Acute toxicity of penitrem A in dogs. Toxicol Appl Pharm 35:311–320
Hayes AW, Phillips RD, Wallace LC (1977) Effect of penitrem A on mouse liver composition. Toxicon 15:293–300
Hayes AW, Hood RD (1978) Effects of prenatal administration of penicillic acid and penitrem A to mice. Toxicon 16:92–96
Cavanagh JB, Holton JL, Nolan CC, Ray DE, Naik JT, Mantle PG (1998) The effects of the tremorgenic mycotoxin penitrem A on the rat cerebellum. Veter Pathol 35:53–63
Walter SL (2002) Acute penitrem A and roquefortine poisoning in a dog. Can Veter J 43:372–374
Do Tat Loi (1991) Nhung cay thuoc va vi thuoc Viet Nam (Glossary of Vietnamese medicinal plants and drugs). Publishing House for Science and Technics, Hanoi, p 165
Perry LM (1980) Medicinal plants of East and Southeast Asia: attributed properties and uses. MIT Press, Cambridge, p 370
Thuy TT, Porzel A, Ripperger H, Van Sung T, Adam G (1999) Bishordeninyl terpene alkaloids from Zanthoxylum avicennae. Phytochemistry 50:903–907
Trinh TT, Tran VS, Adam G (2002) Bishordeninyl terpene alkaloids from Zanthoxylum avicennae. Tap Chi Hoa Hoc 40:41–48
Wang Y, Yong J, Wang Z (2002) Recent progress in bioactive constituents from plants of Zanthoxylum L. Zhongcaoyao 33:666–670
Zhao Y, Cui C, Cai B, Han B, Sun Q (2004) Study on the constituents with anticancer activities of alkaloids from Bauhinia variegata L. Zhongguo Yaowu Huaxue Zazhi 14:169–171
Manandhar NP (2002) Plants and people of Nepal. Timber Press, Oregon
Ibrahim MT, Fobbe R, Nolte J (2004) Chemical composition and biological studies of Egyptian Schinus molle L. and Schinus terebinthifolius raddi oils. Bull Facul Pharm (Cairo University) 42:289–296
Ishii H, Sakurada E, Furukawa T, Koseki C, Ogata K, Koseki N, Ishikawa T, Harayama T (1991) Studies on chemical constituents of rutaceous plants. 71. Photochemistry of 2-(3,4,5-trimethoxyphenyl)-4-(3,4-methylenedioxyphenyl)-4-oxo-2-butenonitrile (β-cyanochalcone): anomalous dimerization through isomerization in the solid state. Chem Pharm Bull 39:2173–2175
Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA (1994) Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricata. Relationship to Fischer indoles and hapalinodoles. J Am Chem Soc 116:9935–9942
Botta B, Misiti D, Delle Monache G, Persichilli S, Vitali A, Botta M, Corelli F, Carmignani M (1997) A multidisciplinary research on Verbesina caracasana. Gazz Chim Ital 127:305–310
Carmignani M, Volpe AR, Delle Monache F, Botta B, Espinal R, De Bonnevaux SC, De Luca C, Botta M, Corelli F, Tafi A, Ripanti G, Delle Monache G (1999) Novel hypotensive agents from Verbesina caracasana. 6. Synthesis and pharmacology of caracasandiamide. J Med Chem 42:3116–3125
Delle Monache G, Botts B, Delle Monache F, Espinal R, De Bonnevaux SC, De Luca C, Botta M, Corelli F, Dei D (1996) Novel hypotensive agents from Verbesina caracasana. 3. Caracasandiamide, a truxinic hypotensive agent from Verbesina caracasana. Bioorg Med Chem Lett 6:233–238
Delle Monache G, Volpe AR, Delle Monache F, Vitali A, Botta B, Espinal R, De Bonnevaux SC, De Luca C, Botta M, Corelli F, Carmignani M (1999) Novel hypotensive agents from Verbesina caracasana. 7. Further hypotensive metabolites from Verbesina caracasana. Bioorg Med Chem Lett 9:3249–3254
Delle Monache G, Botts B, Delle Monache F, Espinal R, De Bonnevaux SC, De Luca C, Botta M, Corelli F, Carmignani M (1992) Caracasanamide, a novel hypotensive agent from Verbesina caracasana. Bioorg Med Chem Lett 25:415–418
Delle Monache G, Botts B, Delle Monache F, Espinal R, De Bonnevaux SC, De Luca C, Botta M, Corelli F, Carmignani M (1993) Novel hypotensive agents from Verbesina caracasana. 2. Synthesis and pharmacology of caracasanamide. Bioorg Med Chem 36:2956–2963
Wildman WC, Pursey BA (1968) Colchicine and related compounds. In: Manske RHF (eds) The alkaloids: chemistry and biology, vol XI. Academic, New York, pp 407–457
Marion BR (1958) Colchicine in agriculture, medicine, biology and chemistry—a review. Br Homoeopat J 47:116–119
Gardner PD, Brandon RL, Haynes GF (1957) The structures of β- and γ-lumicolchicine. ring-D elaboration products. J Am Chem Soc 79:6334–6337
Nagle A, Hur W, Gray NS (2006) Antimitotic agents of natural origin. Curr Drug Target 7:305–326
Le Hello C (1996) La Colchicine. Ann Méd Intern 147:185–211
Sullivan TP (1998) Colchicine in dermatology. J Am Acad Derm 39:993–999
Prescott WA Jr, Johnson CE (2005) Antiinflammatory therapies for cystic fibrosis: past, present, and future. Pharmacother 25:555–573
Richaud-Patin Y, Soto-Vega E, Jakez-Ocampo J, Llorente L (2004) P-glycoprotein in autoimmune diseases. Autoimmun Rev 3:188–192
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nature Rev Cancer 4:253–265
Chappey O, Scherrmann JM (1995) Colchicine: recent data on pharmacokinetics and clinical pharmacology. Rev Med Intern 16:782–789
Chapman OL, Smith HG (1961) The structure of α-lumicolchicine - some examples of diamagnetic shielding by the carbon-oxygen double bond. J Am Chem Soc 83:3914–3916
Alali FQ, Tawaha K, El-Elimat T, Qasaymeh R, Li C, Burgess J, Nakanishi Y, Kroll DJ, Wani MC, Oberlies NH (2006) Phytochemical studies and cytotoxicity evaluations of Colchicum tunicatum Feinbr and Colchicum hierosolymitanum Feinbr (Colchicaceae): 2 Native Jordanian meadow saffrons. Nat Prod Res 20:558–566
Al-Mahmoud MS, Alali FQ, Tawaha K, Qasaymeh RM (2006) Phytochemical study and cytotoxicity evaluation of Colchicum stevenii Kunth (Colchicaceae): a Jordanian meadow saffron. Nat Prod Res 20:153–160
Alali FQ, El-Elimat T, Li C, Qandil A, Alkofahi A, Tawaha K, Burgess JP, Nakanishi Y, Kroll DJ, Navarro HA, Falkinham JO, III; Wani MC, Oberlies NH (2005) New Colchicinoids from a Native Jordanian Meadow Saffron, Colchicum brachyphyllum: isolation of the first naturally occurring dextrorotatory Colchicinoid. J Nat Prod 68:173–178
Ellington E, Bastida J, Viladomat F, Simanek V, Codina C (2003) Occurrence of colchicine derivatives in plants of the genus Androcymbium. Biochem Syst Ecol 31:715–722
Chommadov BCh, Yusupov MK, Aslanov KhA (1991) 2,10-Didemethylcolchicine, a new alkaloid from Merendera robusta. Khim Prirod Soed 1:67–71
Yusupov MK, Chommadov BCh, Aslanov KhA (1991) Homoaporphine alkaloid N-oxides from Merendera raddeana. Khim Prirod Soed 1:86–91
He H, Hu L, Liu F (1999) Chemical constituents of Colchicum autumnale. Huaxue Yanjiu Yu Yingyong 11:509–510
Popova OI, Murav’eva DA, Tolkachev ON (1991) Alkaloids from corms of Colchicum laetum. Khim Prirod Soed 5:731–732
Potesilova H, MacFarlane TD, Guenard D, Simanek V (1987) Alkaloids and phenolics of Wurmbea and Burchardia species. Phytochemistry 26:1031–1032
Turdikulov Kh, Yusupov MK, Sadykov AS (1971) Alkaloids from Colchicum kesselringii bulbs. Khim Prirod Soed 7:541
Yusupov MK, Dinh Thi BN, Aslanov KhA (1975) O-methylkreysigine from Colchicum szovitsii. Khim Prirod Soed 11:526–527
Finnie JF, Van Staden J (1991) Isolation of colchicine from Sandersonia aurantiaca and Gloriosa superba. Variation in the alkaloid levels of plants grown in vivo. J Plant Physiol 138:691–695
Potesilova H, Widermannova J, Santay F (1969) Sustances from the plants of the subfamily Wurmbaeoidease and their derivatives. LXXIII. The lumiderivatives of some colchicine alkaloids and their identification. Coll Czechoslovak Chem Commun 34:3642–3645
Santavy F (1970) Substances from the plants of the subfamily Wurmbaeoideae and their derivatives. LXXIV. Reexamination of some minor alkaloids of unknown composition isolated from Colchicum autumnale. Coll Czechoslovak Chem Commun 35:2857–2860
Thakur RS, Potesilova H, Santavy F (1975) Substances from plants of the subfamily Wurmbaeoideae and their derivatives. LXXIX. Alkaloids of the plant Gloriosa superba. Planta Med 28:201–209
Merchant JR, Joshi V (1976) Chemical constituents of Gloriosa superba Linn. (Liliaceae). Indian J Chem 14B:908
Canonica L, Danieli B, Manitto P, Russo G, Bonati A, Bombardelli E (1969) Structure of β-lumicolchicone. Gazz Chim Ital 99:1059–1067
Dauben WG, Cox DA (1963) Photochemical transformations. XIV. Isocolchicine. J Am Chem Soc 85:2130–2134
Chapman OL, Smith HG, Barks PA (1963) Photoisomerization of isocolchicine. J Am Chem Soc 85:3171–3173
Battersby AR, Reynolds JJ (1960) Biosynthesis of colchicine. Proc Chem Soc 346–347
Battersby AR, Binks R, Yeowell DA (1964) Biosynthesis of colchicine. Proc Chem Soc 86
Battersby AR, Herbert RB (1964) Colchicine. Proc Chem Soc 260
Battersby AR, Binks R, Reynolds JJ, Yeowell DA (1964) Alkaloid biosynthesis. VI. The biosynthesis of colchicine. J Chem Soc 4257–4268
Leete E, Nemeth PE (1960) The biogenesis of the alkaloids of Colchicum. I. The Incorporation of phenylalanine into colchicine. J Am Chem Soc 82:6055–6057
Leete E, Nemeth PE (1961) The biogenesis of the alkaloids of Colchicum. II. Tracer studies with acetate-1-C14 and methionine-methyl-C14. J Am Chem Soc 83:2192–2194
Leete E (1963) The biosynthesis of the alkaloids of Colchicum. III. The incorporation of phenylalanine-2-C14 into colchicine and demecolcine. J Am Chem Soc 85:3666–3669
Leete E (1965) Biosynthesis of the tropolone ring of colchicines. Tetrahedron Lett 6:333–336
Tashkhodzhaev B, Lindeman SV, Bessonova IA, Razakova DM, Tsapkina EN, Struchkov YuT (1988) Haplodimerine, a new dimeric quinoline alkaloid. Khim Prirod Soed 6:838–845
Snieckus VA (1975) Erythrina and related alkaloids. Alkaloids (London) 5:176–182. CA 84:59803
Bates RB, Christensen KA, Hallberg A, Klenck RE, Martin AR (1984) Solid- and liquid-phase photodimerizations of 5H-indolo[1,7-ab] [1] benzazepine. J Org Chem 49:2978–2981
Hallberg A, Isaksson R, Martin AR, Sandstroem J (1989) Chromatographic resolution, circular dichroism spectra, and absolute configurations of dimers of 5H-indolo[1,7-ab] [1] benzazepine and coumarin with C2 symmetry. J Am Chem Soc 111:4387–4392
Ashikaga K, Ito S, Yamamoto M, Nishijima Y (1987) Photodimerization of dibenz[b,f]azepine derivatives and their reaction intermediates. J Photochem 38:321–329
Querner J, Scheller D, Wolff T (2002) Conformational isomers in the photocyclodimerization of N-acylated dibenz[b,f]azepine derivatives. J Photochem Photobiol 150A:85–91
Liu T, Fischer C, Beninga C, Rohr J (2004) Oxidative rearrangement processes in the biosynthesis of gilvocarcin V. J Am Chem Soc 126:12262–12263
McGee LR, Misra R (1990) Gilvocarcin photobiology. Isolation and characterization of the DNA photoadduct. J Am Chem Soc 112:2386–2389
Curini M, Cravotto G, Epifano F, Giannone G (2006) Chemistry and biological activity of natural and synthetic prenyloxycoumarins. Curr Med Chem 13:199–222
Gunaydin K, Savci S (2005) Phytochemical studies on Ruta chalepensis (Lam.) Lamarck. Nat Prod Res 19:203–210
Kavli G, Volden G (1984) Phytophotodermatitis. Photodermatol 1:65–75
Beier RC (1990) Natural pesticides and bioactive components in foods. Rev Environ Cont Toxicol 113:47–137
Cadet J, Voituriez L, Ulrich J, Joshi PC, Wang SY (1984) Isolation and characterization of the mono-heterodimers of 8-methoxypsoralen and thymidine involving the pyrone moiety. Photobiochem Photobiophys 8:35–49
Hahn BS, Joshi PC, Kan LS, Wang SY (1981) Heterodimers of psoralen and thymine derivatives: properties, structure and stereochemistry. Photobiochem Photobiophys 3:113–124
Semmler FW, Mayer EW (1912) Constituents of ethereal oils. Determination of the constitution of active caryophyllene; degradation of active caryophyllene to monocyclic derivatives. Ber Dtsch Chem Ges 44:3657–3679
Semmler FW, Mayer EW (1912) Constituents of ethereal oils. Ber Dtsch Chem Ges 45:3384–3394
Naves YR, Tullen P (1961) Essential oils. CLXXIII. Terpenes from the essential oil of lavender: ocimene, α-pinene, camphene. Helv Chim Acta 44:316–319
Mohamed MAH, Harris PJC, Henderson J (1999) An efficient in vitro regeneration protocol for Tagetes minuta. Plant Cell Tissue Organ Cult 55:211–215
Fromm E, Autin E (1914) Constituents of incense oils. Ann 401:253–262
Austin GH, Baird PD, Chow H-F, Fellows LE, Fleet GWJ, Nash RJ, Peach JM, Pryce RJ, Stirton CH (1987) Isolation from Ateleia herbert-smithii Pittier (Sophoreae, Leguminosae) and X-ray structure of cis-1-amino-3-hydroxymethyl-cyclobutane-1-carboxylic acid, an achiral non-protein amino acid. Tetrahedron 43:1857–1861
Nash RJ (1986) Studies of the chemotaxonomic and ecological significance of secondary compounds in the Leguminosae and Cycadales. Thesis, King’s College, London
Marona HRN, Schenkel EP, Ortega GG, Bergenthal D (1994) Non-proteinogenic amino acids from Ateleia glazioviana Baillon. Rev Ciencias Farm (Sao Paulo) 15:183–195
Marona HRN, Ortega GG, Schenkel EP, Huet J (1996) 1-Amino-3-methylcyclobutane carboxylic acid in seeds of Ateleia glazioviana Baillon (Leguminosae). Acta Farm Bonaer (Brazil) 15:159–162
Ayer SW, Isaac BG, Luchsinger K, Makkar N, Tran M, Stonard RJ (1991) cis-2-Amino-1-hydroxycyclobutane-1-acetic acid, a herbicidal antimetabolite produced by Streptomyces rochei A13018. J Antibiot 44:1460–1462
Pruess DL, Scannell JP, Blount JF, Ax HA, Kellett M, Williams TH, Stempel A (1974) Antimetabolites produced by microorganisms. XI. 1-(S)-hydroxy-2-(S,S)-valylamido-cyclobutane-1-acetic acid. J Antibiot 27:754–759
Pruess D, Scannell JP (1976) Antibiotic X-1092. US Patent: 3939139 19760217; CA 85:3865, p 5
Stoermer R, Laage E (1921) Truxillic acid. V. The seventh acid of the truxillic acid group, neotruxinic acid. Ber Dtsch Chem Ges 54B:96–101
Stoermer R, Bacher F (1922) Configuration of the truxinic and truxillic acids. VI. Ber Dtsch Chem Ges 55B:1860–1882
Adler P (1939) Truxillic acid nitriles and truxillic and truxinic ketones. Sitzber Abhandl Naturforsch Ges Rostock 7:3–20
Stoermer R, Schenk Fr, Pansegrau E (1927) Degradation of the truxillic and truxinic acids. XIII. Ber Dtsch Chem Ges 60B:2566–2591
Stoermer R, Lachmann H (1926) Configuration of β-truxinic acid. XII. Ber Dtsch Chem Ges 59B:642–649
Stoermer R, Scholtz F (1921) Truxillic acid. IV. The 6th acid of the truxillic acid group, ζ-truxinic acid (zetruxinic acid). Ber Dtsch Chem Ges 54B:85–96
Misonou T, Saitoh J, Oshiba S, Tokitomo Y, Maegawa M, Inoue Y, Hori H, Sakurai T (2003) UV-absorbing substance in the red alga Porphyra yezoensis (Bangiales, Rhodophyta) block thymine photodimer production. Mar Biotechnol 5:194–200
Ishihara H (1975) Photodimerization of uracil in water. Kiyo—Nagoya-shiritsu Daigaku Kyoyobu, Shizen Kagaku-hen 21:1–14
Casapullo A, Minale L, Zollo F, Lavayre J (1994) Four new dimeric peptide alkaloids, anchinopeptolides B–D, and cycloanchinopeptolide C, congeners of anchinopeptolide A, from the Mediterranean marine sponge Anchinoe tenacior. J Nat Prod 57:1227–1233
Song F (2001) Solid-state photodimerization of 2-phenylethenyl enamides. Total synthesis of the enamide containing natural products (±)-anchinopeptolide D, (±)-cycloanchinopeptolide D, and (-)-salicylihalamide A. Brandeis University, Waltham, MA, USA. Avail. UMI, Order No. DA3004980. Dissertation 117 p, Dissertation Abstract Int 62:866
Snider BB, Song F, Foxman BM (2000) Total syntheses of (±)-anchinopeptolide D and (±)-cycloanchinopeptolide D. J Org Chem 65:793–800
Walker RP, Faulkner DJ, Van Engen D, Clardy J (1981) Sceptrin, an antimicrobial agent from the sponge Agelas sceptrum. J Am Chem Soc 103:6772–6773
Faulkner DJ (1983) Sceptrin an antimicrobial agent from Agelas sceptrum. US Patent: 4370484 A 19830125, CA 98:155209, p 3
Gaspar H, Gaudencio S, Medeiros MA, Teixeira A, Tavares R, Curto MJM, Devijver C, Braekman JC, Gomez R, De Kluijver M, Van Soest R (2002) Preliminary ecological studies on sponges of the genus Agelas spp. Proc Phytochem Soc Eur 47:495–498
Endo T, Tsuda M, Okada T, Mitsuhashi S, Shima H, Kikuchi K, Mikami Y, Fromont J, Kobayashi J (2004) Nagelamides A–H, new dimeric bromopyrrole alkaloids from marine sponge Agelas species. J Nat Prod 67:1262–1267
Vassas A, Bourdy G, Paillard JJ, Lavayre J, Pais M, Quirion JC, Debitus C (1996) Naturally occurring somatostatin and vasoactive intestinal peptide inhibitors. Isolation of alkaloids from two marine sponges. Planta Med 62:28–30
Bernan VS, Roll DM, Ireland CM, Greenstein M, Maiese WM, Steinberg DA (1993) A study on the mechanism of action of sceptrin, an antimicrobial agent isolated from the South Pacific sponge Agelas mauritiana. J Antimicrob Chemother 32:539–550
Rosa R, Silva W, Escalona de Motta G, Rodriguez AD, Morales JJ, Ortiz M (1992) Antimuscarinic activity of a family of C11N5 compounds isolated from Agelas sponges. Experientia 48:885–887
Keifer PA, Schwartz RE, Koker MES, Hughes RG Jr, Rittschof D, Rinehart KL (1991) Bioactive bromopyrrole metabolites from the Caribbean sponge Agelas conifera. J Org Chem 56:2965–2975
Shen X, Perry TL, Dunbar CD, Kelly-Borges M, Hamann MT (1998) Debromosceptrin, an alkaloid from the Caribbean sponge Agelas conifera. J Nat Prod 61:1302–1303
Kobayashi J, Tsuda M, Ohizumi Y (1991) A potent actomyosin ATP-ase activator from the Okinawan marine sponge Agelas cf. nemoechinata. Experientia 47:301–304
Hao E, Fromont J, Jardine D, Karuso P (2001) Natural products from sponges of the genus Agelas—on the trail of a [2 + 2]-photoaddition enzyme. Molecules 6:130–141
Eder C, Proksch P, Wray V, Van Soest RWM, Ferdinandus E, Pattisina LAS (1999) New bromopyrrole alkaloids from the Indopacific sponge Agelas nakamurai. J Nat Prod 62:1295–1297
Assmann M, Kock M (2002) Bromosceptrin, an alkaloid from the marine sponge Agelas conifera. Z Naturforsch 57C:157–160
Assmann M, Lichte E, Pawlik JR, Kock M (2000) Chemical defenses of the Caribbean sponges Agelas wiedenmayeri and Agelas conifera. Mar Ecol Prog Series 207:255–262
Bickmeyer U (2005) Bromoageliferin and dibromoageliferin, secondary metabolites from the marine sponge Agelas conifera, inhibit voltage-operated, but not store-operated calcium entry in PC12 cells. Toxicon 45:627–632
Barger G (1920) Ergot, its history and chemistry. Pharm J 105:470–473
Flieger M, Wurst M, Shelby R (1997) Ergot alkaloids-sources, structures and analytical methods. Folia Microbiol 42:3–30
Van Dongen PWJ, De Groot ANJA (1995) History of Ergot Alkaloids from Ergotism to Ergometrine, Eur J Obstet. Gynaecol Reprod Biol 60:109–116
Semonsky M, Zikan V (1959) Cycloalkylamides of d-lysergic acid. GB Patent: 816273 19590708, CA 54:7424
Hladovec J, Votava Z (1958) The effect of ergot alkaloids, their partial synthetic derivatives and serotonin on blood clotting. Chekhoslov Fiziol 7:553–558
Macek K, Vanecek S (1962) Ergot alkaloids. XXIV. Paper chromatography of lysergic acid cycloalkamides and N-methylergolinyl-N′-cycloalkylureas. Pharmazie 17:442–444
Votava Z, Podvalova I, Semonsky M (1957) Oxytocic effect of some d-lysergic acid cycloalkyl amides. Nature 179:474–475
Votava Z, Podvalova I, Semonsky M (1958) Pharmacology of D-lysergic acid cycloalkylamides. Archiv Int Pharm Ther 115:114–130
Semonsky M, Zikan V (1959) Cycloalkyl- and ω-cyclopentylalkylamides of d-dihydrolysergic acid. Czech Patent: CS 92350 19591015, CA 56:18477
Holzgrabe U (2005) 200 years of morphine. New developments from research. Pharmaz Z (Germany) 150:32–38
Bogusz MJ (2000) Opiate agonists. In: Bogusz, MJ (ed) Forensic Science (Handbook of analytical separations), vol. 2. Elsevier Science, Amsterdam, pp 3–65.
Stork G (1960) Morphine alkaloids. In: Manske RHF (eds) Alkaloids—chemistry and physiology, vol. 6. Academic, London, pp 219–245
Ghosh AC, Lavoie RL, Herlihy P, Howes JF, Razdan RK (1982) 14-Alkoxy dihydrocodeinones, dihydromorphinones, and morphinanones - a new class of narcotic analgesics. NIDA Res Monograph 41:105–111
Neumeyer JL, Mello NK, Stevens NS, Bidlack JM (2000) Kappa opioid agonists as targets for pharmacotherapies in cocaine abuse. Pharm Acta Helv 74:337–343
Knoll J, Makleit S, Berenyi S, Hosztafi S, Furst Z, Knoll B, Kiss G, Gyulai B (1988) Preparation of N-alkyl-N-demethylazidoethylmorphine derivatives as pharmaceuticals. Hung. Teljes. Hungarian Patent: HU 44554 A2 19880328, CA 109:129414, p 17
Banfield JE, Black DSC, Fallon GD, Gatehouse BM (1983) Constituents of Endiandra species. V. 2-[3′,5′-Dioxo-4′-phenyl-10′-{(E,E)-5″-phenylpenta-2″,4″-dien-1″-yl}-2′,4′,6′-triazatetracyclo [5,4,2,02,6,08,11]tridec-12′-en-9′-yl]acetic acid derived from Endiandra introrsa (Lauraceae). Aust J Chem 36:627–632
Shimada N, Hasegawa S, Harada T, Tomisawa T, Fujii A, Takita T (1986) Oxetanocin, a novel nucleoside from bacteria. J Antibiot 39:1623–1625
Saito S, Hasegawa S, Shimada N (1991) Oxetanocin A manufacture enhancement with Bacillus. Jpn. Kokai Tokkyo Koho, Japanese Patent: JP 03183491 A2 19910809 Heisei. CA 115:206222, p 3
Clement JJ, Kern ER (1991) Cyclobutyl compounds as antiviral agents. Transplant Proceed 23 (Suppl 3):159–161
Hayashi S, Norbeck DW, Rosenbrook W, Fine RL, Matsukura M, Plattner JJ, Broder S, Mitsuya H (1990) Cyclobut-A and cyclobut-G, carbocyclic oxetanocin analogs that inhibit the replication of human immunodeficiency virus in T cells and monocytes and macrophages in vitro. Antimicrob Agents Chemother 34:287–294
Jacobs GA, Tino JA, Zahler R (1989) Synthesis of SQ-32,829, a new nucleoside antiviral agent. Tetrahedron Lett 30:6955–6958
Chiba H, Agematu H, Kaneto R, Terasawa T, Sakai K, Dobashi K, Yoshioka T (1999) Rhodopeptins (Mer-N1033), novel cyclic tetrapeptides with antifungal activity from Rhodococcus sp. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological activities. J Antibiot 52:695–699
Roy O, Faure, Sophie; Aitken, David J (2006) A solution to the component instability problem in the preparation of peptides containing C2-substituted cis-cyclobutane β-aminoacids: synthesis of a stable rhodopeptin analog. Tetrahedron Lett 47:5981–5984
Littman L, Tokar C, Venkatraman S, Roon RJ, Koerner JF, Robinson MB, Johnson RL (1999) Cyclobutane quisqualic acid analogs as selective mGluR5a metabotropic glutamic acid receptor ligands. J Med Chem 42:1639–1647
Gu X, Xian M, Roy-Faure S, Bolte J, Aitken DJ, Gefflaut T (2005) Synthesis of the constrained glutamate analogs (2S,1′R,2′R)- and (2S,1′S,2′S)-2-(2′-carboxycyclobutyl)glycines L-CBG-II and L-CBG-I by enzymatic transamination. Tetrahedron Lett 47:193–196
Lasa M, Lopez P, Cativiela C (2005) Synthesis of the four stereoisomers of cyclobutane analogues of phenylalanine in enantiomerically pure form. Tetrahedron Asym 16:4022–4033
Petschen I, Bosch MP, Guerrero A (2000) Enzyme-catalyzed synthesis and absolute configuration of (1S,2R,5S)- and (1R,2S,5R)-2-(1-hydroxyethyl)-1-(methoxymethyloxyethyl) cyclobutane-1-carbonitrile, key intermediates for the preparation of chiral cyclobutane-containing pheromones. Tetrahedron Asym 11:1691–1695
Izquierdo S, Kogan MJ, Parella T, Moglioni AG, Branchadell V, Giralt E, Ortuno RM (2004) 14-Helical folding in a cyclobutane-containing β-tetrapeptide. J Org Chem 69:5093–5099
Lasa M, Lopez P, Cativiela C (2005) Synthesis of the four stereoisomers of cyclobutane analogues of phenylalanine in enantiomerically pure form. Tetrahedron: Asym 16:4022–4033
Gershonov E, Granoth R, Tzehoval E, Gaoni Y, Fridkin M (1996) 1-Aminocyclobutanecarboxylic acid derivatives as novel structural elements in bioactive peptides: application to tuftsin analogs. J Med Chem 39:4833–4843
Allan RD, Curtis DR, Headley PM, Johnston GAR, Kennedy SME, Lodge D, Twitchin B (1980) Cyclobutane analogs of GABA. Neurochem Res 5:393–400
Moglioni AG, Brousse BN, Alvarez-Larena A, Moltrasio GY, Ortuno RM (2002) Stereoselective synthesis of cyclobutyl GABA analogues and related compounds from (−)-(S)-verbenone. Tetrahedron Asym 13:451–454
Avenoza A, Busto JH, Canal N, Peregrina JM (2005) Synthesis of cyclobutane serine analogues. J Org Chem 70:330–333
Kharkevich DA (1970) Pharmacology of the new antidepolarizing agents, anatruxonium, truxillonium, cyclobutonium, and pyrocyclonium. In: Kharkevich DA (ed) Novue kurarepodobnye ganglioblokiruyushchie stredstava. Meditsina, Moscow, pp 41–48
Kharkevich DA, Skoldinov AP, Arendaruk AP, Kazakova TP, Muratov VK (1974) Anatruxonium as a new curarelike agent of nondepolarizing action. Khim Farmatsev Zh (USSR) 8:59–62
Kharkevich DA (1965) Pharmacological properties of the new curaroid, anatruxonium. Farmakol Toksikol (Moscow) 28:305–309
Kosuge S, Hayashi M, Hamanaka N (1982) Synthesis of thromboxane A2 analog, DL-(9,11)-methano-(11,12)-aminothromboxane A2. Tetrahedron Lett 23:4027–4030
Chung S-K, Ban SH, Woo SH (1995) Heterocyclic lipids with PAF antagonist activities 3. Synthesis of 2,4-bis(hydroxymethyl)-oxetane and 1,3-bis(hydroxymethyl) cyclobutane derivatives. Korean J Med Chem 5:84–93
Fuerst JA (2005) Intracellular compartmentation in planctomycetes. Ann Rev Microbiol 59:299–328
Fuerst JA (1995) The planctomycetes: emerging models for microbial ecology, evolution and cell biology. Microbiol 141:1493–1506
Kim BS, Moon SS, Hwang BK (2000) Structure elucidation and antifungal activity of an anthracycline antibiotic, Daunomycin, isolated from Actinomadura roseola. J Agric Food Chem 48:1875–1881
Cassinelli G, Rivola G, Ruggieri D, Arcamone F, Grein A, Merli S, Spalla C, Casazza AM, Di Marco A, Pratesi G (1982) New anthracycline glycosides: 4-O-demethyl-11-deoxydoxorubicin and analogues from Streptomyces peucetius var. aureus. J Antibiot (Tokyo) 35:176–83
Gauze GF, Sveshnikova MA, Ukholina RS, Gavrilina GN, Filicheva VA, Gladkikh EG (1973) Production of antitumor antibiotic carminomycin by Actinomadura carminata sp. nov. Antibiot (USSR) 18:675–678
Gauze GF, Terekhova LP, Maksimova TS, Ol’khovatova OL, Lavrova NV (1975) New producer of carminomycin, Actinomycer cremeospinus sp. nov. Antibiot (USSR) 20:389–393
Smith TH, Fujiwara AN, Lee WW, Wu HY, Henry DW (1977) Synthetic approaches to adriamycin. 2. Degradation of daunorubicin to a nonasymmetric tetracyclic ketone and refunctionalization of the a ring to adriamycin. J Org Chem 42:3653–3660
Tevyashova A, Sztaricskai F, Batta G, Herczegh P, Jeney A (2004) Formation of squaric acid amides of anthracycline antibiotics. Synthesis and cytotoxic properties. Bioorg Med Chem Lett 14:4783–4789
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dembitsky, V.M. Bioactive cyclobutane-containing alkaloids. J Nat Med 62, 1–33 (2008). https://doi.org/10.1007/s11418-007-0166-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-007-0166-3