Abstract
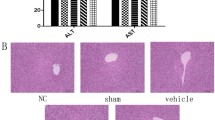
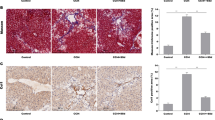
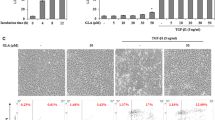
The present study was designed to investigate the effects of betulinic acid on human hepatic stellate cells in vitro and C57BL/6 mice in vivo, as well as the signaling pathways involved. In this study, we explored the effects of betulinic acid on expression of alpha smooth muscle actin and autophagy-related proteins. Betulinic acid reduced pathological damage associated with liver fibrosis, as well as serum platelet-derived growth factor and serum hydroxyproline levels. Furthermore, betulinic acid downregulated the expression of alpha smooth muscle actin and type I collagen in mouse liver and upregulated the expression of microtubule-associated protein light chain 3B and autophagy-related gene 7 at the gene and protein levels. LC3II expression was increased and alpha smooth muscle actin expression was decreased in betulinic acid-treated hepatic stellate cells. Interventions with bafilomycin A1 and mCherry-GFP-LC3 adenoviruses promoted the formation of autophagosomes in hepatic stellate cells and the development of autophagic flow. Our study found that mitogen-activated protein kinase/extracellular signal-regulated kinase may be involved in the effects of betulinic acid on liver fibrosis. The present study suggests that betulinic acid has anti-hepatic fibrosis activity by inducing autophagy and could serve as a promising new agent for treating hepatic fibrosis.





Similar content being viewed by others
Abbreviations
- BA:
-
Betulinic acid
- PDGF:
-
Platelet-derived growth factor
- 3-MA:
-
3-Methyladenine
- BafA1:
-
Bafilomycin A1
- TEM:
-
Transmission electron microscopy
- PBS:
-
Phosphate-buffered saline
- MAPK:
-
Mitogen-activated protein kinase
- P70S6K:
-
Ribosomal protein S6 kinase
- ERK:
-
Extracellular-regulated protein kinases
- Col:
-
Colchicine
- LC3:
-
Microtubule-associated protein 1 light chain 3
- ATG7:
-
Autophagy-related protein 7
- α-SMA:
-
Alpha smooth muscle actin antibody
- COL-1:
-
Collagen I
- PI3K:
-
Phosphatidylinositol 3-kinase
- mTOR:
-
The mammalian target of rapamycin
- ECM:
-
Extracellular matrix
References
Schuppan D, Pinzani M (2012) Anti-fibrotic therapy: lost in translation? J Hepatol 56(Suppl 1):S66–S74. https://doi.org/10.1016/S0168-8278(12)60008-7
Kubow KE, Vukmirovic R, Zhe L, Klotzsch E, Smith ML, Gourdon D, Luna S, Vogel V (2015) Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat Commun 6:8026. https://doi.org/10.1038/ncomms9026
Zou X, Ramachandran P, Kendall TJ, Pellicoro A, Dora E, Aucott RL, Manwani K, Man TY, Chapman KE, Henderson NC, Forbes SJ, Webster SP, Iredale JP, Walker BR, Michailidou Z (2018) 11Beta-hydroxysteroid dehydrogenase-1 deficiency or inhibition enhances hepatic myofibroblast activation in murine liver fibrosis. Hepatology (Baltimore, Md.) 67:2167–2181. https://doi.org/10.1002/hep.29734
Fujioka T, Kashiwada Y, Kilkuskie RE, Cosentino LM, Ballas LM, Jiang JB, Janzen WP, Chen IS, Lee KH (1994) Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzygium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod 57:243–247
Damle AA, Pawar YP, Narkar AA (2013) Anticancer activity of betulinic acid on MCF-7 tumors in nude mice. Indian J Exp Biol 51:485–491
Ahangarpour A, Shabani R, Farbood Y (2018) The effect of betulinic acid on leptin, adiponectin, hepatic enzyme levels and lipid profiles in streptozotocin-nicotinamide-induced diabetic mice. Res Pharm Sci 13:142–148. https://doi.org/10.4103/1735-5362.223796
Yi J, Xia W, Wu J, Yuan L, Wu J, Tu D, Fang J, Tan Z (2014) Betulinic acid prevents alcohol-induced liver damage by improving the antioxidant system in mice. J Vet Sci 15:141–148
Brusotti G, Montanari R, Capelli D, Cattaneo G, Laghezza A, Tortorella P, Loiodice F, Peiretti F, Bonardo B, Paiardini A, Calleri E, Pochetti G (2017) Betulinic acid is a PPARgamma antagonist that improves glucose uptake, promotes osteogenesis and inhibits adipogenesis. Sci Rep 7:5777. https://doi.org/10.1038/s41598-017-05666-6
Seo J, Jung J, Jang DS, Kim J, Kim JH (2017) Induction of cell death by betulinic acid through induction of apoptosis and inhibition of autophagic flux in microglia BV-2 cells. Biomol Ther (Seoul) 25:618–624. https://doi.org/10.4062/biomolther.2016.255
Yang LJ, Chen Y, He J, Yi S, Wen L, Zhao J, Zhang BP, Cui GH (2012) Betulinic acid inhibits autophagic flux and induces apoptosis in human multiple myeloma cells in vitro. Acta Pharmacol Sin 33:1542–1548. https://doi.org/10.1038/aps.2012.102
Vural A, Kehrl JH (2014) Autophagy in macrophages: impacting inflammation and bacterial infection. Scientifica (Cairo) 2014:825463. https://doi.org/10.1155/2014/825463
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075. https://doi.org/10.1038/nature06639
Itakura E, Mizushima N (2010) Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy 6:764–776
Kroemer G (2015) Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest 125:1–4. https://doi.org/10.1172/JCI78652
Luo X, Wang D, Zhu X, Wang G, You Y, Ning Z, Li Y, Jin S, Huang Y, Hu Y, Chen T, Meng Y, Li X (2018) Autophagic degradation of caveolin-1 promotes liver sinusoidal endothelial cells defenestration. Cell Death Dis 9:576. https://doi.org/10.1038/s41419-018-0567-0
Lodder J, Denaes T, Chobert MN, Wan J, El-Benna J, Pawlotsky JM, Lotersztajn S, Teixeira-Clerc F (2015) Macrophage autophagy protects against liver fibrosis in mice. Autophagy 11:1280–1292. https://doi.org/10.1080/15548627.2015.1058473
Wang K, Damjanov I, Wan YY (2009) The protective role of pregnane X receptor in lipopolysaccharide/d-galactosamine-induced acute liver injury. Lab Invest J Tech Methods Pathol 90:257–265. https://doi.org/10.1038/labinvest.2009.129
Liu H, Zhang Z, Hu H, Zhang C, Niu M, Li R, Wang J, Bai Z, Xiao X (2018) Protective effects of Liuweiwuling tablets on carbon tetrachloride-induced hepatic fibrosis in rats. BMC Complementary Altern Med 18:212. https://doi.org/10.1186/s12906-018-2276-8
Zhang K, Gao Y, Zhong M, Xu Y, Li J, Chen Y, Duan X, Zhu H (2016) Hepatoprotective effects of Dicliptera chinensis polysaccharides on dimethylnitrosamine-induced hepatic fibrosis rats and its underlying mechanism. J Ethnopharmacol 179:38–44. https://doi.org/10.1016/j.jep.2015.12.053
Lin X, Xu W, Shao M, Fan Q, Wen G, Li C, Jing L, Sun X (2015) Shenling Baizhu San supresses colitis associated colorectal cancer through inhibition of epithelial-mesenchymal transition and myeloid-derived suppressor infiltration. BMC Complementary Altern Med 15:126. https://doi.org/10.1186/s12906-015-0649-9
Zhang F, Kong D, Zhang Z, Lei N, Zhu X, Zhang X, Chen L, Lu Y, Zheng S (2013) Tetramethylpyrazine induces G0/G1 cell cycle arrest and stimulates mitochondrial-mediated and caspase-dependent apoptosis through modulating ERK/p53 signaling in hepatic stellate cells in vitro. Apoptosis 18:135–149. https://doi.org/10.1007/s10495-012-0791-5
Yeganeh B, Ghavami S, Kroeker AL, Mahood TH, Stelmack GL, Klonisch T, Coombs KM, Halayko AJ (2015) Suppression of influenza A virus replication in human lung epithelial cells by noncytotoxic concentrations bafilomycin A1. Am J Physiol Lung Cell Mol Physiol 308:L270–L286. https://doi.org/10.1152/ajplung.00011.2014
Yokoyama T, Kondo Y, Kondo S (2007) Roles of mTOR and STAT3 in autophagy induced by telomere 3′ overhang-specific DNA oligonucleotides. Autophagy 3:496–498
Javadov S, Jang S, Agostini B (2014) Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: therapeutic perspectives. Pharmacol Ther 144:202–225. https://doi.org/10.1016/j.pharmthera.2014.05.013
Martinez-Lopez N, Singh R (2014) ATGs: scaffolds for MAPK/ERK signaling. Autophagy 10:535–537. https://doi.org/10.4161/auto.27642
Torok NJ (2008) Recent advances in the pathogenesis and diagnosis of liver fibrosis. J Gastroenterol 43:315–321. https://doi.org/10.1007/s00535-008-2181-x
Yang N, Dang S, Shi J, Wu F, Li M, Zhang X, Li Y, Jia X, Zhai S (2017) Caffeic acid phenethyl ester attenuates liver fibrosis via inhibition of TGF-beta1/Smad3 pathway and induction of autophagy pathway. Biochem Biophys Res Commun 486:22–28. https://doi.org/10.1016/j.bbrc.2017.02.057
Pal A, Ganguly A, Chowdhuri S, Yousuf M, Ghosh A, Barui AK, Kotcherlakota R, Adhikari S, Banerjee R (2015) Bis-arylidene oxindole-betulinic acid conjugate: a fluorescent cancer cell detector with potent anticancer activity. ACS Med Chem Lett 6:612–616. https://doi.org/10.1021/acsmedchemlett.5b00095
Oyebanji BO, Saba AB, Oridupa OA (2014) Studies on the anti-inflammatory, analgesic and antipyrexic activities of betulinic acid derived from Tetracera potatoria. Afr J Tradit Complement Altern Med 11:30–33
Diedrich D, Wildner AC, Silveira TF, Silva G, Santos FD, Da SE, Do CV, Visioli F, Gosmann G, Bergold AM, Zimmer AR, Netz PA, Gnoatto S (2018) SERCA plays a crucial role in the toxicity of a betulinic acid derivative with potential antimalarial activity. Chem Biol Interact 287:70–77. https://doi.org/10.1016/j.cbi.2018.03.014
Innocente AM, Silva GN, Cruz LN, Moraes MS, Nakabashi M, Sonnet P, Gosmann G, Garcia CR, Gnoatto SC (2012) Synthesis and antiplasmodial activity of betulinic acid and ursolic acid analogues. Molecules 17:12003–12014. https://doi.org/10.3390/molecules171012003
Ulukaya S, Basturk B, Kilic M, Ulukaya E (2008) Cytokine gene polymorphism and postreperfusion syndrome during orthotopic liver transplantation. Transplant Proc 40:1290–1293. https://doi.org/10.1016/j.transproceed.2008.01.078
Schneider JL, Cuervo AM (2014) Liver autophagy: much more than just taking out the trash. Nat Rev Gastroenterol Hepatol 11:187–200. https://doi.org/10.1038/nrgastro.2013.211
Chen JW, Ni BB, Li B, Yang YH, Jiang SD, Jiang LS (2014) The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cell Physiol Biochem 34:1175–1189. https://doi.org/10.1159/000366330
Zhao J, Peng L, Cui R, Guo X, Yan M (2016) Dimethyl alpha-ketoglutarate reduces CCl4-induced liver fibrosis through inhibition of autophagy in hepatic stellate cells. Biochem Biophys Res Commun 481:90–96. https://doi.org/10.1016/j.bbrc.2016.11.010
Opipari AJ, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR (2004) Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res 64:696–703
Gu X, Wang Y, Xiang J, Chen Z, Wang L, Lu L, Qian S (2013) Interferon-gamma triggers hepatic stellate cell-mediated immune regulation through MEK/ERK signaling pathway. Clin Dev Immunol 2013:389807. https://doi.org/10.1155/2013/389807
Huang Q, Liu X, Cao C, Lei J, Han D, Chen G, Yu J, Chen L, Lv D, Li Z (2016) Apelin-13 induces autophagy in hepatoma HepG2 cells through ERK1/2 signaling pathway-dependent upregulation of Beclin1. Oncol Lett 11:1051–1056. https://doi.org/10.3892/ol.2015.3991
Yue X, Zhao P, Wu K, Huang J, Zhang W, Wu Y, Liang X, He X (2016) GRIM-19 inhibition induced autophagy through activation of ERK and HIF-1alpha not STAT3 in Hela cells. Tumour Biol 37:9789–9796. https://doi.org/10.1007/s13277-016-4877-5
Suzuki K, Hino M, Kutsuna H, Hato F, Sakamoto C, Takahashi T, Tatsumi N, Kitagawa S (2001) Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1beta. J Immunol 167:5940–5947
Zhang L, Wang H, Zhu J, Xu J, Ding K (2014) Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem Biophys Res Commun 450:247–254. https://doi.org/10.1016/j.bbrc.2014.05.101
Ko CP, Lin CW, Chen MK, Yang SF, Chiou HL, Hsieh MJ (2015) Pterostilbene induce autophagy on human oral cancer cells through modulation of Akt and mitogen-activated protein kinase pathway. Oral Oncol 51:593–601. https://doi.org/10.1016/j.oraloncology.2015.03.007
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81603501 and 81774170), Science and Technology Planning Project of Guangdong Province (2014A020221097), Administration of Traditional Chinese Medicine of Guangdong Province (20162087), Science and Technology Planning Project of Guangzhou City (201508020014 and 201707010080), the Natural Science Foundation of Guangdong Province (2018B030306012 and 2017A030313903), the Scientific Research Initiative Program of Southern Medical University (LX2015N003 and CX2017N001), Combined Science Technology Project of Guangdong Provincial Department of Science and Technology and Guangdong Provincial Academy of Traditional Chinese Medicine (2014A020221011); Guangdong Province Bureau of Traditional Chinese Medicine Scientific Research Project (20161161).
Author information
Authors and Affiliations
Contributions
ZL and XS participated in the conception and design of the study; YL and YB participated in generation, collection, assembly, and interpretation of data; CM and TZ participated in drafting and revision of the manuscript; SH and LG participated in statistical analysis; ZL obtained funding.
Corresponding authors
Ethics declarations
Conflict of interest statement
The authors have no disclosures to report.
Rights and permissions
About this article
Cite this article
Liu, Y., Bi, Y., Mo, C. et al. Betulinic acid attenuates liver fibrosis by inducing autophagy via the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway. J Nat Med 73, 179–189 (2019). https://doi.org/10.1007/s11418-018-1262-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1262-2