Abstract
Purpose
The recreational drug market remains dynamic. After the introduction of 25I-NBOH, an N-benzylphenethylamine and new psychoactive substance, as option for LSD and NBOMe drugs, new NBOH substances have been identified in recent years. Herein, we report our efforts for the identification and structural elucidation of three new NBOHs detected in seized blotter papers: 25B-NBOH, 25C-NBOH, and 25E-NBOH.
Methods
Blotter papers seized between 2017 and 2018 by local police force in Brazil were submitted to extraction, purification, identification and characterization using attenuated total reflectance-Fourier transform infrared spectroscopy, gas chromatography—mass spectrometry, liquid chromatography—tandem mass spectrometry, and one- and two-dimensional nuclear magnetic resonance spectroscopy.
Results
Three new NBOHs were characterized: 2-(((4-ethyl-2,5-dimethoxyphenethyl)amino)methyl)phenol (25E-NBOH, 2C-E-NBOH), 2-(((4-chloro-2,5-dimethoxyphenethyl)amino)methyl)phenol (25C-NBOH, 2C-C-NBOH), and 2-(((4-bromo-2,5-dimethoxyphenethyl)amino)methyl)phenol (25B-NBOH, 2C-B-NBOH).
Conclusions
To our knowledge, this is the first report for identification and detailed characterization of 25B-NBOH, 25C-NBOH, and 25E-NBOH in seized samples. NBOH substances are not under United Nations Conventions control. The identification of seized blotter papers between 2014 and beginning of 2019 showed that NBOH substances have become the main hallucinogenic drug in the region. These group are thermolabile under gas chromatographic conditions, demanding other analytical approaches of analysis to avoid misidentifications. Unfortunately, the knowledge about toxicology of NBOHs are limited.
Similar content being viewed by others
Introduction
New psychoactive substances (NPS) have become a global and continuously growing phenomenon over the last decade, posing a risk to public health while remaining a challenge to law enforcement agencies [1, 2]. From 2009 to 2017, over 800 NPS have been reported in 111 countries and territories, most of them being classified as phenethylamines, synthetic cathinones or synthetic cannabinoids [3].
Phenethylamines are agonists of the serotonin (5-HT, 5-hydroxytryptamine) receptors [4]. The N-benzyl substitution in phenethylamines (usually from the 2C-X family, such as 2C-I and 2C-B) improve the binding and functional activity in 5-HT receptors, with N-(2-hydroxybenzyl) substituted substances usually presenting the highest affinities to the \(\hbox {5-HT}_{\mathrm{2A}}\) receptor [4,5,6].
Since 2015, a new NPS group based on N-benzyl-2,5-dimethoxyphenethylamines (Fig. 1), commonly described as NBOHs, emerged as recreational drugs. NBOHs, substances structurally closely related to NBOMes, are usually found on blotter papers and have become an option for LSD (lysergic acid diethylamide) users. Self-reported use of NBOHs has been reported in United States [7], Australia [8], and Germany [9]. These substances were already identified for street samples seized in Brazil [10], Finland, Germany, and Slovenia [11].
Like most NPS, knowledge about the toxicology of NBOHs is very limited or virtually nonexistent. According to information gathered from user forums (not to be considered as canon), the effects include tachycardia, and auditory and visual hallucinations [12, 13]. While both NBOHs and NBOMes apparently taste bitter, thus providing a simple way for users to distinguish them from LSD [14], NBOHs are reported to induce weaker effects and to last less than NBOMes, being unaccompanied with “body load”, an unpleasant physical sensation reported by drug users [14].
Together with usual difficulties associated with the identification of NPS (unknown substance, never before reported, not present in reference spectral libraries, no certified standards available, etc), detection of NBOHs present yet another important trait: thermal degradation under standard gas chromatography, which seriously increases the risk of misidentification [15, 16] and demands the development of other detection methods [17,18,19,20,21].
Herein, we report our efforts for the identification and structural elucidation of three new NBOHs detected in seized samples submitted to our forensic laboratory: 25B-NBOH, 25C-NBOH and 25E-NBOH.
Materials and methods
Chemicals and reagents
Acetone, chloroform, diethyl ether, methanol, sodium hydroxide, and hydrochloric acid were purchased from Synth (Diadema, SP, Brazil); methanol LC grade from Merck (Darmstadt, Germany); ammonium formate and methanol-\(\textit{d}_{4}\) with 99.8% isotopic purity, containing 0.03% (v/v) tetramethylsilane (TMS), from Sigma-Aldrich (St. Louis, MO, USA); the ultrapure water from Milipore Direct Q3 (Billerica, MA, USA); silica gel 60 (70–230 mesh) from Merck.
Samples and extraction
Blotter papers suspected to contain illicit substances were seized between 2017 and 2018 by police forces from Minas Gerais. These blotters usually represented a colorful side including drawings of cartoon and game characters, scientists, deities, etc. and a white side (Fig. 2). The samples were grouped according to previous routine analysis, such as gas chromatography–mass spectrometry (GC–MS) and liquid chromatography–tandem mass spectrometry (LC–MS/MS), as follows: one hundred blotter papers for samples named as 01 and 02, and twenty-five blotter papers for sample named as 03.
Each sample group (01, 02, and 03) was submitted to three successive extractions: 30 mL of chloroform (2 \(\times\)) and 30 mL of methanol in beakers by 20 min in magnetic stirrer. After mixing the 90 mL of solvent extracted, 1 mL of NaOH solution (1%, w/v) was added. Each solution was evaporated to dryness getting a solid reddish residue. Each residue was submitted to column chromatography using silica gel as stationary phase. The elution was performed with chloroform, acetone, and methanol, in increasing polarity order, providing the corresponding fractions. The acetone fraction was evaporated to dryness and 20 mL of saturated solution of hydrochloric acid in diethyl ether was added to the residue, providing, after new evaporation to dryness, a beige solid labeled as compound 01 (15.1 mg), compound 02 (13.3 mg), and compound 03 (4.5 mg).
Instrumentation
Attenuated total reflectance Fourier transform infrared spectroscopy analysis
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) analyses were performed directly from the solids, except for compound 03 due to the small amount available. They were obtained using a NicoletTM iZ10 spectrometer equipped with EverGlo infrared (IR) source, DLaTGS room temperature IR detector under \(\hbox {N}_2\) purge and single-bounce Smart \(\hbox {Orbit}^{\mathrm{TM}}\) accessory module with diamond ATR crystal (Thermo Fischer Scientific, Madison, WI, USA). Each spectrum was averaged over 16 scans, taken at 4 cm\(^{-1}\) resolution, with maximum detector window aperture and minimum interferometer mirror speed, in the range of 400–4000 cm\(^{-1}\).
Gas chromatography–mass spectrometry analysis
Samples were prepared by transferring 1 mg of solid to a microcentrifuge tube and adding 1 mL of LC grade methanol. Each solution was transferred to a vial for analysis. Analysis were performed on an Agilent GC 7890A (Agilent Technologies, Santa Clara, CA, USA) coupled with an Agilent XL MSD 5975C quadrupole mass spectrometer. The chromatographic separation was performed with a 433 HP-1MS capillary column (30 m \(\times\) 0.25 mm, i.d., film thickness 0.25 \(\upmu\)m; Agilent Technologies). A volume of 1 \(\upmu\)L of the sample solution was injected with a split ratio of 20:1. Helium was used as carrier gas with a constant flow of 1.0 mL min\(^{-1}\). The temperature of injector and the GC–MS interface was set at 280 and \(300\,^{\circ }\hbox {C}\), respectively. The oven temperature was initially maintained at \(150\,^{\circ }\hbox {C}\) for 1.5 min; increased to \(250\,^{\circ }\hbox {C}\) at \(30\,^{\circ }\hbox {C min}^{-1}\) and maintained for 1 min; increased to \(300\,^{\circ }\hbox {C}\) at \(50\,^{\circ }\hbox {C min}^{-1}\) and maintained for 3 min (total run time: 11.8 min). The solvent delay was set to 1.5 min. The mass scan range was set to m/z 35–550.
Liquid chromatography coupled to tandem mass spectrometry analysis
LC–MS/MS analyses were performed on a Prominence UFLC system (Shimadzu Corporation, Kyoto, Japan) coupled with a Shimadzu LCMS 8030 triple quadrupole mass spectrometer with electrospray ionization source (ESI) set in the positive ionization mode. LabSolutionsTM (Shimadzu Corporation) was used for data acquisition and processing.
Direct flow injections were performed with a total flow rate of 0.4 mL min\(^{-1}\). The mobile phase consisted of 50% of ultra-pure water (A) and 50% of acetonitrile (B), both containing ammonium formate (0.024%, w/v). The injection volume was 1 \(\upmu\)L and the total run time was 1 min.
The MS conditions were as follows: desolvation line temperature, \(250\,^{\circ }\hbox {C}\); heat block temperature, \(400\,^{\circ }\hbox {C}\); nebulizing gas flow, 3 L \(\hbox {min}^{-1}\); drying gas flow, \(\hbox {15 L min}^{-1}\); collision-induced dissociation (CID) gas pressure, 230 kPa; dwell time, 100 ms; and interface voltage, 4.5 kV. A Q3 full scan were set from m/z 110 to 500 to seek out the protonated molecule ([M+H]\(^{+}\)), and product ion scan (PIS) were performed with collision energies of − 10, − 20, − 30, and − 40 eV for the purpose of analyzing the fragmentation pattern.
Nuclear magnetic ressonance spectroscopy analysis
The nuclear magnetic resonance (NMR) experiment was conducted using 12 mg of compound 01, 12 mg of compound 02 and 3.5 mg of compound 03. Each solid was dissolved in 0.6 mL methanol-\(\textit{d}_{4}\) with tetramethylsilane (TMS) as internal standard. \(^{1}\)H, \(^{13}\)C, DEPT 135 (Distortionless Enhancement by Polarization Transfer), HSQC (Heteronuclear Single Quantum Coherence) and HMBC (Heteronuclear Multiple-Bond Correlation) experiments were recorded using a Bruker Avance DRX 600 MHz spectrometer (Bruker, Ettlingen, Germany). Acquisition was conducted by TopSpin software 4.0.6 version (Bruker) and processing was conduct by MestReNova 12.0 (MestreLab Research, Santiago de Compostela, Spain).
Results
ATR-FTIR analysis
ATR-FTIR spectra of compounds 01 and 02 indicated the presence of small impurities that were not eliminated during the purification processes. The single infrared spectral profile analysis using available forensic libraries (SWGDRUG and Europe Response infrared library) indicated presence of signals matching with NBOH drugs [22, 23]. Interpretation of the ATR-FTIR spectra indicated the same findings from our previous work [15]: the higher degree of similarity with 25R-NBOMe when compared with 2C-R and a systematic decrease of the spectral band centered around 1250 cm\(^{-1}\), characteristic of asymmetric C–O–C vibrations of NBOMe compounds, indicating the substitution of \(\hbox {OCH}_3\) by OH (Fig. 3).
GC–MS analysis
The GC–MS analysis of compounds 01, 02 and 03 (Fig. 4) indicated the presence of, respectively, 2C-E (RT: retention time = 4.58 min), 2C-C (RT = 4.85 min) and 2C-B (RT = 5.15 min) as most intense peaks according to mass spectra library available (Table 1). The mass spectra for the secondary peaks (RTs = 2.33, 2.34, and 2.31 min, respectively; Fig. 5) were all similar but they had no reasonable match on MS libraries currently. These results have already been predicted for other NBOHs corroborating the finding that this group of substances are thermolabile under the GC temperature conditions [16]. Considering the results of mass spectrum libraries and the presence of secondary peaks, there was an indication that substances 01, 02 and 03 were 25E-NBOH, 25C-NBOH, and 25B-NBOH, respectively. The thermal fragmentation reactions and proposed mass fragmentation pathways for these substances are presented in Fig. 6 according to the previous works [16, 24].
LC–MS/MS analysis
The positive full scan and PIS mass spectra of compounds 01, 02, and 03 are presented in Fig. 7. For compound 02, the relative intensity of the ion at m/z 322 was 3:1 of signal at m/z 324. For compound 03, the relative intensity of the ion at m/z 368 was 1:1 of signal at m/z 370. These isotopologue patterns indicate the presence of chlorine and bromine atoms in chemical structures of 02 and 03, respectively. The precursor ion and product ions of the analyzed substances (Fig. 8) are compatible with mass fragmentation pathway proposed in the previous works [15, 25] and can be summarized as shown in Table 2.
NMR analysis
NMR spectroscopic results of 01, 02, and 03 are presented in Tables 3, 4, and 5, respectively. Minor impurities were detected in the three spectra without prejudice to the data interpretation. All experiments (\(^{1}\)H, \(^{13}\)C, DEPT 135, HSQC and HMBC) were used for characterizing 01 and 02. However, due to the limited amount of 03, \(^{13}\)C signals were assigned only by HSQC and HMBC experiments.



It was observed thirteen types of H-signal (two of these signals were overlapped) and nineteen types of C-signal for compound 01; eleven types of H-signal and seventeen types of C-signal for compound 02 and 03. In general, these signals, supported by HSQC and HMBC experiments, were attributed to one 1,2,4,5-tetra-substituted aromatic ring containing two methoxy, one ethylmethylamine group and an ethyl (01), chlorine (02) or bromine group (03). It was observed that the amine group was attached to one (2-hydroxyphenyl)methyl group. Based on structural elucidation using NMR, compound 01 was identified as 2-(((4-ethyl-2,5-dimethoxyphenethyl)amino)methyl)phenol (25E-NBOH, 2C-E-NBOH), compound 02 as 2-(((4-chloro-2,5-dimethoxyphenethyl)amino)methyl)phenol (25C-NBOH, 2C-C-NBOH), and compound 03 as 2-(((4-bromo-2,5-dimethoxyphenethyl)amino)methyl)phenol (25B-NBOH, 2C-B-NBOH). These data were in agreement with those observed by ATR-FTIR, GC–MS and LC–MS/MS.
NBOH outlook
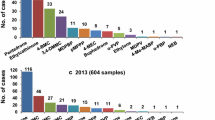
The number of identifications of N-benzylphenethylamines (NBOMes and NBOHs) and LSD by our laboratory in seized samples between 2014 and the first semester of 2019 is shown in Fig. 9a, updating our previous work [10]. NBOMes dominated the blotter paper drug market up to the beginning of 2016. Starting at this point, NBOH identification has increased, overcoming NBOMes at the second semester of 2016. In contrast, the number of NBOMes identifications has decreased and are, nowadays, less significant than LSD (305 identifications of NBOHs, 86 of LSD and 20 of NBOMes, between July 2018 and June 2019).
Changes of N-benzylphenethylamine identification between first trimester (T) of 2014 and second trimester of 2019 (a) and NBOH identification between third trimester of 2015 and second trimester of 2019 (b). Asterisk: local ban of several NBOMe substances; double asterisk: local ban of 25I-NBOH; triple asterisk: local ban of other NBOH substances
From 2015 to 2017, the only NBOH detected was 25I-NBOH. The other NBOHs (Fig. 9b) started appearing in the last two years, with 25B-NBOH first identified in 2017, and 25C-NBOH and 25E-NBOH in 2018, totalizing 376 NBOHs identifications in blotter paper (Jan 2017 to Jun 2019). Although 25H-NBOH has been also identified in 2017, it was considered primarily as a minor component, always associated with other major component NBOHs, and was not included in the number of identifications considered until January 2018.
Discussion
To our knowledge, the present study is the first academical work to identify and to characterize 25B-NBOH, 25C-NBOH, and 25E-NBOH in authentic street seized samples. With the addition of 25I-NBOH, the first substance of these group reported, NBOHs has become a consolidated option in the recreational drug market. Regarding the legal aspect, none of NBOH substances are controlled by any United Nations Conventions. Due to the efforts of local forensic laboratories, some NBOHs are scheduled in Brazil as prohibited substances: 25I-NBOH (October 2016), 25B-NBOH, 25C-NBOH, 25E-NBOH and 25H-NBOH (December 2018) [26]. Scheduling of the N-benzylphenethylamines seems to have impact in local drug market, namely decreasing the availability of scheduled drugs. The dynamics of such process, specially its rate, however, is not clearly enough; while an NBOH was first identified in a seized sample about 1.5 years after the ban of the corresponding NBOMe, newer NBOHs emerged only seven months after the ban of 25I-NBOH. Although these numbers help us to illustrate the dynamics of the drug market, they should not be considered accurately because they are significantly influenced by the agility of police investigation, seizures of drug materials and the ability of forensic laboratories to establish the identity of new substances.
The knowledge about pharmacology and toxicology of these NBOHs, as well others NPS, are limited. 25I-NBOH showed a higher selectivity for the \(\hbox {5-HT}_{\mathrm{2A}}\) receptor when compared to \(\hbox {5-HT}_{\mathrm{2C}}\) (449.8-fold higher) followed by 25C-NBOH (43.7-fold higher), 25E-NBOH (31.0-fold higher), and 25B-NBOH (8.7-fold higher) [5]. Many studies on toxicokinetics and toxicodynamics of NBOHs remain to be explored.
Conclusions
The permanent emergence of NPS is, undoubtedly, a result of the efforts of drug dealers in circumventing drug controls. In this context, here we have presented data on the identification and structural characterization of three NPS, members of the NBOH class: 25B-NBOH, 25C-NBOH and 25E-NBOH. To our knowledge, this is the first report to identify and to characterize these three substances in authentic street seized samples which have emerged in the Brazilian market after 25I-NBOH was scheduled. As predicted in a previously published work, we have confirmed that these three substances are thermolabile, degrading into the corresponding 2C-X molecule during its injection in standard gas chromatography, easily leading them to be misidentified as such, a grave error from the forensic point of view.
References
United Nations Office on Drugs and Crime (UNODC) (2017) World drug report 2017—market analysis of synthetic drugs: amphetamine-type stimulants, new psychoactive substances. https://www.unodc.org/documents/scientific/Booklet_4_Market_Analysis_of_Synthetic_Drugs_ATS_NPS.pdf. Accessed 10 Jul 2019
United Nations Office on Drugs and Crime (UNODC) (2018a) Global SMART update—understanding the synthetic drug market: the NPS factor. https://www.unodc.org/documents/scientific/Global_Drugs_Assessment_2017.pdf. Accessed 17 Aug 2018
United Nations Office on Drugs and Crime (UNODC) (2018b) World drug report 2018—analysis of drug markets: opiates, cocaine, cannabis, synthetic drugs. https://www.unodc.org/wdr2018/prelaunch/WDR18_Booklet_3_DRUG_MARKETS.pdf. Accessed 15 Apr 2019
Nichols DE (2012) Structure–activity relationships of serotonin \(\text{5-HT }_{{\rm 2A}}\) agonists. WIREs Membr Transp Signal 1:559–579. https://doi.org/10.1002/wmts.42 (open access article)
Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (2014) Synthesis and structure–activity relationships of N-benzyl phenethylamines as \(\text{5-HT }_{{\rm 2A/2C}}\) agonists. ACS Chem Neurosci 5:243–249. https://doi.org/10.1021/cn400216u
Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Johnson RA, Janowsky A (2018) Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: high potency agonists at \(\text{5-HT }_{{\rm 2A}}\) receptors. Biochem Pharmacol 158:27–34. https://doi.org/10.1016/j.bcp.2018.09.024
Palamar JJ, Barratt MJ, Ferris JA, Winstock AR (2016) Correlates of new psychoactive substance use among a self-selected sample of nightclub attendees in the United States. Am J Addict 25:400–407. https://doi.org/10.1111/ajad.12403
Sutherland R, Bruno R, Peacock A, Dietze P, Breen C, Burns L, Barratt MJ (2017) New psychoactive substances: purchasing and supply patterns in Australia. Hum Psychopharmacol Clin Exp 32:e2577. https://doi.org/10.1002/hup.2577 (open access article)
Hannemann T-V, Kraus L, Piontek D (2017) Consumption patterns of nightlife attendees in Munich: a latent-class analysis. Subst Use Misuse 52:1511–1521. https://doi.org/10.1080/10826084.2017.1290115 (open access article)
Machado Y, Coelho Neto J, Lordeiro RA, Silva MF, Piccin E (2019) Profile of new psychoactive substances (NPS) and other synthetic drugs in seized materials analysed in a Brazilian forensic laboratory. Forensic Toxicol 37:265–271. https://doi.org/10.1007/s11419-018-0456-3
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2017) EMCDDA—Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA. https://doi.org/10.2810/430586
Drugs-Forum (2009) 25I-NBOH Experiences. https://drugs-forum.com/threads/25i-nboh-experiences.95632. Accessed 2 May 2019
Reddit (2014) NBOMe vs. NBOH. https://www.reddit.com/r/Drugs/comments/1wj3v0/nbome_vs_nboh. Accessed 15 Apr 2019
Psychedelic Dad (2014) NBOH and NBOMe series. http://psychedelicdad.blogspot.com/2014/05/nboh-and-nbome-series.html. Accessed 15 Apr 2019
Arantes LC, Júnior EF, de Souza LF, Cardoso AC, Alcântara TLF, Lião LM, Machado Y, Lordeiro RA, Coelho Neto J, Andrade AFB (2017) 25I-NBOH: a new potent serotonin \(\text{5-HT }_{{\rm 2A}}\) receptor agonist identified in blotter paper seizures in Brazil. Forensic Toxicol 35:408–414. https://doi.org/10.1007/s11419-017-0357-x (open access article)
Coelho Neto J, Andrade AFB, Lordeiro RA, Machado Y, Elie M, Ferrari Júnior E, Arantes LC (2017) Preventing misidentification of 25I-NBOH as 2C-I on routine GC–MS analyses. Forensic Toxicol 35:415–420. https://doi.org/10.1007/s11419-017-0362-0
de Morais DR, Barbosa IL, Cunha KF, Tripodi GL, Angolini CFF, Franco MF, de Aquino EM, Eberlin MN, Costa JL (2017) EASI-IMS an expedite and secure technique to screen for 25I-NBOH in blotter papers. J Mass Spectrom 52:701–706. https://doi.org/10.1002/jms.3977
Souza GA, Arantes LC, Guedes TJ, de Oliveira AC, Marinho PA, Muñoz RAA, dos Santos WTP (2017) Voltammetric signatures of 2,5-dimethoxy-N-(2-methoxybenzyl) phenethylamines on boron-doped diamond electrodes: detection in blotting paper samples. Electrochem Commun 82:121–124. https://doi.org/10.1016/j.elecom.2017.08.001
Magalhães LdO, Arantes LC, Braga JWB (2019) Identification of NBOMe and NBOH in blotter papers using a handheld NIR spectrometer and chemometric methods. Microchem J 144:151–158. https://doi.org/10.1016/j.microc.2018.08.051
Ng XQ, Hamzah A, Ling M, Goh E, Moy HY, Yao YJ, Lui CP (2019) Qualitative analysis of synthetic opioids, synthetic hallucinogens and LSD in urine using LC–MS/MS. Aust J Forensic Sci 51:S229–S233. https://doi.org/10.1080/00450618.2019.1568565
de Andrade AFB, Gonzalez-Rodriguez J (2019) Electroanalytical identification of 25I-NBOH and 2C-I via differential pulse voltammetry: a rapid and sensitive screening method to avoid misidentification. Analyst 144:2965–2972. https://doi.org/10.1039/c9an00062c
Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG) (2019) SWGDRUG IR library version 2.0. http://www.swgdrug.org/ir.htm. Accessed 10 Mar 2019
Republic of Slovenia, Ministry of the Interior Police (2017) Response ATR-IR library. https://www.policija.si/eng/index.php/generalpolicedirectorate/1669-nfl-page-response. Accessed 5 Aug 2018
Zuba D, Sekuła K (2013) Identification and characterization of 2,5-dimethoxy-3,4-dimethyl-\(\beta\)-phenethylamine (2C-G)—a new designer drug. Drug Test Anal 5:549–559. https://doi.org/10.1002/dta.1396
Chia XWS, Ong MC, Yeo YYC, Ho YJ, Nasir EIBA, Tan L-LJ, Chua PY, Yap TWA, Lim JLW (2019) Simultaneous analysis of 2Cs, 25-NBOHs, 25-NBOMes and LSD in seized exhibits using liquid chromatography—tandem mass spectrometry: a targeted approach. Forensic Sci Int 301:394–401. https://doi.org/10.1016/j.forsciint.2019.05.036
Agência Nacional de Vigilância Sanitária (ANVISA) (2019) Lista de substâncias sujeitas a controle especial no Brasil. http://portal.anvisa.gov.br/lista-de-substancias-sujeitas-a-controle-especial. Accessed 2 Apr 2019
Acknowledgements
We would like to thank all our laboratory colleagues and Ms. Ivana Silva Lula (Departamento de Quí-mica, Universidade Federal de Minas Gerais) by carry out NMR experiments. José Coelho Neto is supported by FIP-PUC-MG ( Fundo de Incentivo à Pesquisa da Pontifícia Universidade Católica de Minas Gerais). Rosemeire Brondi Alves and Evandro Piccin are supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) research fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Machado, Y., Coelho Neto, J., Lordeiro, R.A. et al. Identification of new NBOH drugs in seized blotter papers: 25B-NBOH, 25C-NBOH, and 25E-NBOH. Forensic Toxicol 38, 203–215 (2020). https://doi.org/10.1007/s11419-019-00509-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-019-00509-7