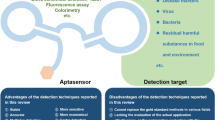
Abstract
Aptamers are a series of high-affinity and high-specificity oligoneucleotides (single-stranded DNA or RNA) to the target, usually selected by the combinatorial chemistry SELEX technique (systematic evolution of ligands by exponential enrichment). Aptamers have proved to be one kind of novel functional molecules in life science and chemistry. After being labeled by signaling groups, the aptamer probe can conveniently transfer the characteristics of aptamer-target recognition to a form of high-sensitive signal, and the high-affinity, high-specificity measurements of metal ion, organic molecules, nucleic acid, proteins, or cells become possible. This article summarizes the recent advances of aptamer probes in different sensing fields, with special emphasis on aptamer probes as fluorescent sensors.
Similar content being viewed by others
References
Ellington A D, Szostak J W. In vitro selection of RNA molecules that bind specific ligands. Nature, 1990, 346(6287): 818–822
Jenison R D, Gill S C, Pardi A, Polisky B. High-resolution molecular discrimination by RNA. Science, 1994, 263(5152): 1425–1429
Geiger A, Burgstaller P, von der Eltz H, Roeder A, Famulok M. RNA aptamers that bind L-arginine with sub-micromolar dissociation constants and high enantioselectivity. Nucleic Acids Res, 1996, 24(6): 1029–1036
Yang Q, Goldstein I J, Mei H Y, Engelke D R. DNA ligands that bind tightly and selectively to cellobiose. Proc Natl Acad Sci USA, 1998, 95(10): 5462–5467
Ruckman J, Green L S, Beeson J, Waugh S, Gillette W L, Henninger D D, Claesson-Welsh L, Janjić N. 2-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor(VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem, 1998, 273(32): 20556–20557
Shangguan D, Li Y, Tang Z, Cao Z C, Chen H W, Mallikaratchy P, Sefah K, Yang C J, Tan W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Soc USA, 2006, 103(32): 11838–11843
Tang J, Xie J, Shao N, Yan Y. The DNA aptamers that specifically recognize ricin toxin are selected by two in vitro selection methods. Electrophoresis, 2006, 27(7): 1303–1311
Gokulrangan G, Unruh J R, Holub D F, Ingram B, Johnson C K, Wilson G S. DNA aptamer-based bioanalysis of IgE by fluorescence anisotropy. Anal Chem, 2005, 77(7): 1963–1970
Okazawa A, Maeda H, Fukusaki E, Katakura Y, Kobayashi A. In vitro selection of hematoporphyrin binding DNA aptamers. Bioorg Med Chem Lett, 2000, 10(23): 2653–2656
Jhaveri S D, Kirby R, Conrad R, Maglott E J, Bowser M, Kennedy R T, Glick G, Ellington A.D. Designed signaling aptamers that transduce molecular recognition to changes in fluorescence intensity. J Am Chem Soc, 2000, 122(11): 2469–2473
Yamana K, Ohtani Y, Nakano H, Saito I. Bis-pyrene labeled DNA aptamer as an intelligent fluorescent biosensor. Bioorg Med Chem Lett, 2003, 13(20): 3429–3431
Merino E J, Weeks K M. Facile conversion of aptamers into sensors using a 2′-ribose-linked fluorophore. J Am Chem Soc, 2005, 127(37): 12766–12767
Katilius E, Katiliene Z, Woodbury N W. Signaling aptamers created using fluorescent nucleotide analogues. Anal Chem, 2006, 78(18): 6484–6489
Jiang Y, Fang X, Bai C. Signaling aptamer/protein binding by a molecular light switch complex. Anal Chem, 2004, 76(27): 5230–5235
Tang J, Yu T, Guo L, Xie J, Shao N, He Z. In vitro selection of DNA aptamer against abrin toxin and aptamer-based abrin direct detection. Biosens Bioelectron, 2007, 22(11): 2456–2463
Li B, Wei H, Dong S. Sensitive detection of protein by an aptamer-based label-free fluorescing molecular switch. Chem Commun, 2007, 29(1): 73–75
Jiang Y, Zhou C, Fang X. Aptamer-based ATP assay using a luminescent light switching complex. Anal Chem, 2005, 77(11): 3542–3546
Nutiu R, Li Y. Structure-switching signaling aptamers. J Am Chem Soc, 2003, 125(16): 4771–4778
Nutiu R, Li Y. Aptamers with fluorescence-signaling properties. Methods, 2005, 37(1): 16–25
Heyduk E, Heyduk T. Nucleic acid-based fluorescence sensors for detecting proteins. Anal Chem, 2005, 77(4): 1147–1156
Choi J H, Chen K H, Strano M S. Aptamer-capped nanocrystal quantum dots: A new method for label-free protein detection. J Am Chem Soc, 2006, 128(49): 15584–15585
Ikanovic M, Rudzinski W E, Bruno J G, Allman A, Carrillo M P, Dwarakanath S, Bhahdigadi S, Rao P, Kiel J L, Andrews C J. Fluorescence assay based on aptamer-quantum dot binding to Bacillus thuringiensis spores. J Fluoresc, 2007, 17(2): 193–199
Liu J, Lee J H, Lu Y. Quantum Dot encoding of aptamer-linked nanostructures for one-pot simultaneous detection of multiple analytes. Anal Chem, 2007, 79(11): 4120–4125
Yang C J, Jockusch S, Vicens M, Turro N J, Tan W. Light-switching excimer probes for rapid protein monitoring in complex biological fluids. Proc. Natl Acad Sci USA, 2005, 102(48): 17278–17283
Nagatoishi S, Nojima T, Juskowiak B, Takenaka S. A pyrene-labeled G-quadruplex oligonucleotide as a fluorescent probe for potassium ion detection in biological applications. Angew Chem Int Ed, 2005, 44(32): 5067–5070
Cao Z, Tan W. Molecular aptamers for real-time protein-protein interaction study. Chem Eur J, 2005, 11(15): 4502–4508
Nutiu R, Yu J M, Li Y. Signaling aptamers for monitoring enzymatic activity and for inhibitor screening. Chem Bio Chem, 2004, 5(8): 1139–1144
Pavlov V, Shlyahovsky B, Willner I. Fluorescence detection of DNA by the catalytic activation of an aptamer/thrombin complex. J Am Chem Soc, 2005, 127(18): 6522–6523
Hartig J S, Najafi-Shoushtari S H, Grüne I, Yan A, Ellington A D, Famulok M. Protein-dependent ribozymes report molecular interactions in real time. Nat Biotechnol, 2003, 20(7): 717–722
Srinivasan J, Cload S T, Hamaguchi N, Kurz J, Keene S, Kurz M, Boomer R M, Blanchard J, Epstein D, Wilson C, Diener J L. ADP-specific sensors enable universal assay of protein kinase activity. Chem Biol, 2004, 11(4): 499–508
Seetharaman S, Zivarts M, Sudarsan N, Breaker R R. Immobilized RNA switches for the analysis of complex chemical and biological mixtures. Nat Biotechnol, 2001, 19(4): 336–341
Yang L, Fung C W, Cho E J, Ellington A D. Real-time rolling circle amplification for protein detection. Anal Chem, 2007, 79(9): 3320–3329
Di Giusto D A, Wlassoff W A, Gooding J J, Messerle B A, King G C. Proximity extension of circular DNA aptamers with real-time protein detection. Nucleic Acids Res, 2005, 33(6): e64
Cho E J, Yang L, Levy M, Ellington A D. Using a deoxyribozyme ligase and rolling circle amplification to detect a non-nucleic acid analyte, ATP. J Am Chem Soc, 2005, 127(7): 2022–2023
Shlyahovsky B, Li D, Weizmann Y, Nowarski R, Kotler M, Willner I. Spotlighting of cocaine by an autonomous aptamer-based machine. J Am Chem Soc, 2007, 129(13): 3814–3815
Huang C C, Huang Y F, Cao Z, Tan W, Chang H T. Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal Chem, 2005, 77(17): 5735–5741
Wang L, Liu X, Hu X, Song S, Fan C. Unmodified gold nanoparticles as a colorimetric probefor potassium DNA aptamers. Chem Commun, 2006, 28(36): 3780–3782
Zhao W, Chiuman W, Brook M A, Li Y. Simple and rapid colorimetric biosensors based on DNA aptamer and noncrosslinking gold nanoparticle aggregation. Chem Bio Chem, 2007, 8(7): 727–731
Liu J, Lu Y. Fast colorimetric sensing of adenosine and cocaine based on a general sensor design involving aptamers and nanoparticles. Angew Chem Int Ed, 2006, 45(1): 90–94
Blank M, Weinschenk T, Priemer M, Schluesener H. Systematic evolution of a DNA aptamer binding to rat brain tumor microvessels-selective targeting of endothelial regulatory protein pigpen. J Biol Chem, 2001, 276(19): 16464–16468
Smith J E, Medley C D, Tang Z, Shangguan D, Lofton C, Tan W. Aptamer-conjugated nanoparticles for the collection and detection of multiple cancer cells. Anal Chem, 2007, 79(8): 3075–3082
Lupold S E, Hicke B J, Lin Y, Coffey D S. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res, 2002, 62(14): 4029–4033
Chu T C, Shieh F, Lavery L A, Levy M, Richards-Kortum R, Korgel B A, Ellington A D. Labeling tumor cells with fluorescent nanocrystal-aptamer bioconjugates. Biosens Bioelectron, 2006, 21(10): 1859–1866
Farokhzad O C, Jon S, Khademhosseini A, Tran T T, LaVan D A, Langer R. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res, 2004, 64(21): 7668–7672
Farokhzad O C, Cheng J, Teply B A, Sherifi I, Jon S, Kantoff P W, Richie J P, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA, 2006, 103(16): 6315–6320
Cheng J, Teply B A, Sherifi I, Sung J, Luther G, Gu F X, Levy-Nissenbaum E, Radovic-Moreno A F, Langer R, Farokhzad O C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials, 2007, 28(5): 869–876
Baker B R, Lai R Y, Wood M S, Doctor E H, Heeger A J, Plaxco K W. An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J Am Chem Soc, 2006, 128(10): 3138–3139
Lai R Y, Plaxco K W, Heeger A J. Aptamer-based electrochemical detection of picomolar platelet-derived growth factor directly in blood serum. Anal Chem, 2007, 79(1): 229–233
Wu Z, Guo M, Zhang S, Chen C, Jiang J, Shen G, Yu R. Reusable electrochemical sensing platform for highly sensitive detection of small molecules based on structure-switching signaling aptamers. Anal Chem, 2007, 79(7): 2933–2939
Zuo X, Song S, Zhang J, Pan D, Wang L, Fan C. A target-responsive electrochemical aptamer switch (TREAS) for reagentless detection of nanomolar ATP. J Am Chem Soc, 2007, 129(5): 1042–1043
Hansen J A, Wang J, Kawde A, Xiang Y, Gothelf K V, Collins G. Quantum-dot/aptamer-based ultrasensitive multi-analyte electrochemical biosensor. J Am Chem Soc, 2006, 128(7): 2228–2229
Centi S, Tombelli S, Minunni M, Mascini M. Aptamer-based detec tion of plasma proteins by an electrochemical assay coupled to magnetic beads. Anal Chem, 2007, 79(4): 1466–1473
Polsky R, Gill R, Kaganovsky L, Willner I. Nucleic acid-functionalized Pt nanoparticles: Catalytic labels for the amplified electrochemical detection of biomolecules. Anal Chem, 2006, 78(7): 2268–2271
Le Floch F, Ho H A, Leclerc M. Label-free electrochemical detection of protein based on a ferrocene-bearing cationic polythiophene and aptamer. Anal Chem, 2006, 78(13): 4727–4731
Ho H A, Doré K, Boissinot M, Bergeron M G, Tanguay R M, Boudreau D, Leclerc M. Direct molecular detection of nucleic acids by fluorescence signal amplification. J Am Chem Soc, 2005, 127(36): 12673–12676
Xu D, Xu D, Yu X, Liu Z, he W, Ma Z. Label-free electrochemical detection for aptamer-based array electrodes. Anal Chem, 2005, 77(16): 5107–5113
Radi A, Acero Sánchez J L, Baldrich E, O’sullivan C K. Reusable impedimetric aptasensor. Anal Chem, 2005, 77(19): 6320–6323
Zayats M, Huang Y, Gill R, Ma C, Willner I. Label-free and reagentless aptamer-based sensors for small molecules. J Am Chem Soc, 2006, 128(42): 13666–13667
Willner I, Zayats M. Electronic aptamer-based sensors. Angew Chem Int Ed, 2007, 46(34): 6408–6418
Minunni M, Tombelli S, Gullotto A, Luzi E, Mascini M. Development of biosensors with aptamers as bio-recognition element: the case of HIV-1 Tat protein. Biosens Bioelectron, 2004, 20(6): 1149–1156
Schlensog M D, Gronewold T M A, Tewes M, Famulok M, Quandt E. A love-wave biosensor using nucleic acids as ligands. Sens Actuators B, 2004, 101(3): 308–315
Win M N, Klein J S, Smolke C D. Codeine-binding RNA aptamers and rapid determination of their binding constants using a direct coupling surface plasmon resonance assay. Nucleic Acids Res., 2006, 34(19): 5670–5682
Li Y, Lee H J, Corn R M. Detection of protein biomarkers using RNA aptamer microarrays and enzymatically amplified surface plasmon resonance imaging. Anal Chem, 2007, 79(3): 1082–1088
Wlotzka B, Leva S, Eschgfäller B, Burmeister J, Kleinjung F, Kaduk C, Muhn P, Hess-Stumpp H, Klussmann S. In vivo properties of an anti-GnRH spiegelmer: An example of an oligonucleotide-based therapeutic substance class. Proc Natl Acad Sci USA, 2002, 99(13): 8898–8902
Stoltenburg R, Reinemann C, Strehlitz B. SELEX-A (r) evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng, 2007, 24(4): 381–403
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 20575078 and 20705039)
Rights and permissions
About this article
Cite this article
Li, Y., Guo, L., Zhang, Z. et al. Recent advances of aptamer sensors. Sci. China Ser. B-Chem. 51, 193–204 (2008). https://doi.org/10.1007/s11426-008-0001-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0001-z