Abstract
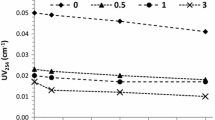
During the chlorine disinfection of reclaimed-water, the proportion of bromo-disinfection by-products (bromo-DBPs) in total DBPs is affected by chlorine dosage, reaction time, pH, ammonia nitrogen (NH3-N) and preozonation. Results show that bromo-trihalomethanes (bromo-THMs) form more easily than bromo-haloacetic acids (bromo-HAAs) and bromine incorporation in DBPs decreases with the increase of chlorine dosage. Within 5 h, bromine incorporation in THMs (n(Br)) increases but bromine incorporation in HAAs (n′(Br)) decreases with the extension of reaction time; however, n(Br) decreases and n′(Br) keeps relatively constant at a longer reaction time. Furthermore, bromine incorporation in DBPs is low under acidic and alkaline conditions. The increase of NH3-N concentration inhibits the formation of chloro-DBPs, resulting in the increase of n(Br) and n′(Br) to some extent. Preozonation enhances the formation of HOBr and the increase of bromine incorporation in DBPs; however, ozone of a high concentration oxidizes HOBr to its salt form, leading to the decrease of bromine incorporation in DBPs.
Similar content being viewed by others
References
Trevizo C, Nirmalak H N. Prediction of microbial toxicity of industrial organic chemicals. Water Sci Technol, 1999, 39(10–11): 63–69
Zha J M, Wang Z J. Assessing technological feasibility for wastewater reclamation based on early life stage toxicity of Japanese medaka (Oryzias latipes). Agricul Ecos Environ, 2005, 107(2–3): 187–198
Wong G T F, Davidson J A. The fate of chlorine in sea-water. Water Res, 1977, 11(11): 971–978
Symons J M, Kranser S W, Simms L A, Selimenti M. Measurement of THM and precursor concentrations revisited: The effect of bromide ion. J Am Water Wks Assoc, 1993, 85(1): 51–62
Chang E E, Lin Y P, Chiang P C. Effects of bromide on the formation of THMs and HAAs. Chemosphere, 2001, 43(8): 1029–1034
Uyak V, Toroz I. Investigation of bromide ion effects on disinfection by-products formation and speciation in an Istanbul water supply. J Hazard Mater, 2007, 149(2): 445–451
Wu W, Chadik P. Effect of bromide ion on haloacetic acid formation during chlorination of a Biscayne aquifer water. J Environ Eng, 1998, 124(10): 392–398
Warne M A, Osborn D, Lindon J C, Nicholson J K. Quantitative structure toxicity relationship for halogenated substituted-benzenes to vibrio fischeri, using atoms-based semi-empirical molecular-orbital descriptors. Chemosphere, 1999, 38(14): 3357–3382
Nobukawa T, Sanukida S. The genotoxicity of by-products by chlorination and ozonation of the river water in the presence of bromide ions. Water Sci Technol, 2000, 42(3–4): 259–264
Richardson S D. Disinfection by-products and other emerging contaminants in drinking water. TrAC-Trends Anal Chem, 2003, 22(10):666–684
Chellam S, Krasner S W. Disinfection byproducts relationships and speciation in chlorinated nanofiltered waters. Environ Sci Technol, 2001, 35(19): 3988–3999
Shon H K, Vigneswaran S, Snyder S A. Effluent organic matter (EfOM) in wastewater: Constituents, effects, and treatment. Crit Rev Env Sci Technol, 2006, 36(4): 327–374
Wert E C, Rosario-Ortiz F L D, Drury D D. Formation of oxidation byproducts from ozonation of wastewater. Water Res, 2007, 41(7):1481–1490
Yang X, Chii Shang C, Huang J C. DBP formation in breakpoint chlorination of wastewater. Water Res, 2005, 39(19): 4755–4767
Michalowicz J, Duda W, Stufka-Olczyk J. Transformation of phenol, catechol, guaiacol and syringol exposed to sodium hypochlorite. Chemosphere, 2007, 66(4): 657–663
Yang X, Shang C, Westerhoff P. Factors affecting formation of haloacetonitriles, haloketones, chloropicrin and cyanogen halides during chloramination. Water Res, 2007, 41(6): 1193–1200
Wang L S, Hu H Y, Wang C. Effect of ammonia nitrogen and dissolved organic matter fractions on the genotoxicity of wastewater effluent during chlorine disinfection. Environ Sci Technol, 2007, 41(1):160–165
Cowman G A, Singer P C. Effect of bromide ion on haloacetic acid speciation resulting from chlorination and chloramination of aquatic humic substances. Environ Sci Technol, 1996, 30(1): 16–24
Zhang X R, Echigo S, Lei H X, Smith M E, Minear R A, Talley J W. Effects of temperature and chemical addition on the formation of bromoorganic DBPs during ozonation. Water Res, 2005, 39(2–3):423–435
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 50538090) and Funds for Creative Research Groups of China (Grant No. 50621804)
Rights and permissions
About this article
Cite this article
Zhang, H., Qu, J., Liu, H. et al. Proportion of bromo-DBPs in total DBPs during reclaimed-water chlorination and its related influencing factors. Sci. China Ser. B-Chem. 51, 1000–1008 (2008). https://doi.org/10.1007/s11426-008-0097-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0097-1