Abstract
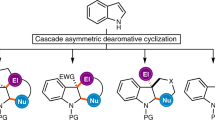
The first enantioselective Beckwith-Enholm cyclization reaction is reported herein. Under cooperative photoredox and chiral hydrogen-bonding catalysis mediated by visible light, cyclization of carbonyls with azaarene-based olefins as a new reaction system offers a general and divergent synthetic pathway to furnish a variety of highly valuable enantioenriched azaarene-functionalized carbocyclic and heterocyclic alcohols, which bear adjacent 1,2- or nonadjacent 1,3-stereocentres on distinct cyclic frameworks, in high yields and enantio- and diastereoselectivities. The good compatibility of various azaarenes and carbonyls as well as the diversity of cyclic structures of the products underscores the generality of the catalysis platform. In addition to the ability to precisely introduce deuterium into molecules in an enantioselective manner, the considerable synthetic value of this method includes the excellent antioxidant stress potential of the products. In particular, molecule 29 was determined to be a promising lead compound for antioxidant stress drug design.

Similar content being viewed by others
References
Streuff J. Synthesis, 2013, 45: 281–307
Ardisson J, Férézou JP, Julia M, Pancrazi A. Tetrahedron Lett, 1987, 28: 2001–2003
Corey EJ, Pyne SG. Tetrahedron Lett, 1983, 24: 2821–2824
Yeh CH, Korivi RP, Cheng CH. Adv Synth Catal, 2013, 355: 1338–1344
Beckwith ALJ, Roberts DH. J Am Chem Soc, 1986, 108: 5893–5901
Sugawara T, Otter BA, Ueda T. Tetrahedron Lett, 1988, 29: 75–78
Porter NA, Chang VHT, Magnin DR, Wright BT. J Am Chem Soc, 1988, 110: 3554–3560
Enholm EJ, Prasad G. Tetrahedron Lett, 1989, 30: 4939–4942
Enholm EJ, Burroff JA. Tetrahedron Lett, 1992, 33: 1835–1838
Hays DS, Fu GC. J Org Chem, 1996, 61: 4–5
Mikami K, Yamaoka M. Tetrahedron Lett, 1998, 39: 4501–4504
Tripp JC, Schiesser CH, Curran DP. J Am Chem Soc, 2005, 127: 5518–5527
Hays DS, Fu GC. J Org Chem, 1998, 63: 6375–6381
Otsubo K, Inanaga J, Yamaguchi M. Tetrahedron Lett, 1986, 27: 5763–5764
Molander GA, Kenny C. J Am Chem Soc, 1989, 111: 8236–8246
Corey EJ, Zheng GZ. Tetrahedron Lett, 1997, 38: 2045–2048
Estévez RE, Oller-López JL, Robles R, Melgarejo CR, Gansäuer A, Cuerva JM, Oltra JE. Org Lett, 2006, 8: 5433–5436
Shono T, Ohmizu H, Kawakami S, Sugiyama H. Tetrahedron Lett, 1980, 21: 5029–5032
Cossy J, Belotti D, Cuong NK, Chassagnard C. Tetrahedron, 1993, 49: 7691–7700
Cossy J, Belotti D. Tetrahedron, 2006, 62: 6459–6470
Tarantino KT, Liu P, Knowles RR. J Am Chem Soc, 2013, 135: 10022–10025
Fava E, Nakajima M, Nguyen ALP, Rueping M. J Org Chem, 2016, 81: 6959–6964
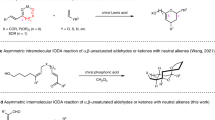
Wang ZS, Chen YB, Wang K, Xu Z, Ye LW. Green Chem, 2020, 22: 4483–4488
Prier CK, Rankic DA, MacMillan DWC. ChemRev, 2013, 113: 5322–5363
Shaw MH, Twilton J, MacMillan DWC. J Org Chem, 2016, 81: 6898–6926
Romero NA, Nicewicz DA. Chem Rev, 2016, 116: 10075–10166
Chen Y, Lu LQ, Yu DG, Zhu CJ, Xiao WJ. Sci China Chem, 2019, 62: 24–57
Zhang HH, Chen H, Zhu C, Yu S. Sci China Chem, 2020, 63: 637–647
Mangelinckx S, Giubellina N, De Kimpe N. Chem Rev, 2004, 104: 2353–2400
Best D, Lam HW. J Org Chem, 2014, 79: 831–845
Evano G, Theunissen C. Angew Chem Int Ed, 2019, 58: 7558–7598
Proctor RSJ, Phipps RJ. Angew Chem Int Ed, 2019, 58: 13666–13699
Zhao Y, Xia W. Org Biomol Chem, 2019, 17: 4951–4963
Yin Y, Zhao X, Jiang Z. ChemCatChem, 2020, 12: 4471–4489
Miyazawa K, Yasu Y, Koike T, Akita M. Chem Commun, 2013, 49: 7249–7251
Lee KN, Lei Z, Ngai MY. J Am Chem Soc, 2017, 139: 5003–5006
Lima F, Sharma UK, Grunenberg L, Saha D, Johannsen S, Sedelmeier J, Van der Eycken EV, Ley SV. Angew Chem Int Ed, 2017, 56: 15136–15140
Trowbridge A, Reich D, Gaunt MJ. Nature, 2018, 561: 522–527
Yin Y, Dai Y, Jia H, Li J, Bu L, Qiao B, Zhao X, Jiang Z. J Am Chem Soc, 2018, 140: 6083–6087
Cao K, Tan SM, Lee R, Yang S, Jia H, Zhao X, Qiao B, Jiang Z. JAm Chem Soc, 2019, 141: 5437–5443
Yin Y, Li Y, Gonçalves TP, Zhan Q, Wang G, Zhao X, Qiao B, Huang KW, Jiang Z. J Am Chem Soc, 2020, 142: 19451–19456
Kong M, Tan Y, Zhao X, Qiao B, Tan CH, Cao S, Jiang Z. JAm Chem Soc, 2021, 143: 4024–4031
Wang C, Lu Z. Org Chem Front, 2015, 2: 179–190
Brimioulle R, Lenhart D, Maturi MM, Bach T. Angew Chem Int Ed, 2015, 54: 3872–3890
Jiang C, Chen W, Zheng WH, Lu H. Org Biomol Chem, 2019, 17: 8673–8689
Yin Y, Zhao X, Qiao B, Jiang Z. Org Chem Front, 2020, 7: 1283–1296
Lv X, Xu H, Yin Y, Zhao X, Jiang Z. Chin J Chem, 2020, 38: 1480–1488
Hepburn HB, Melchiorre P. Chem Commun, 2016, 52: 3520–3523
Proctor RSJ, Davis HJ, Phipps RJ. Science, 2018, 360: 419–422
Liu X, Liu Y, Chai G, Qiao B, Zhao X, Jiang Z. Org Lett, 2018, 20: 6298–6301
Qiao B, Li C, Zhao X, Yin Y, Jiang Z. Chem Commun, 2019, 55: 7534–7537
Fu MC, Shang R, Zhao B, Wang B, Fu Y. Science, 2019, 363: 1429–1434
Zheng D, Studer A. Angew Chem Int Ed, 2019, 58: 15803–15807
Bauer A, Westkämper F, Grimme S, Bach T. Nature, 2005, 436: 1139–1140
Rono LJ, Yayla HG, Wang DY, Armstrong MF, Knowles RR. J Am Chem Soc, 2013, 135: 17735–17738
Liu Y, Li J, Ye X, Zhao X, Jiang Z. Chem Commun, 2016, 52: 13955–13958
Lin L, Bai X, Ye X, Zhao X, Tan CH, Jiang Z. Angew Chem Int Ed, 2017, 56: 13842–13846
Li J, Kong M, Qiao B, Lee R, Zhao X, Jiang Z. Nat Commun, 2018, 9: 2445
Roos CB, Demaerel J, Graff DE, Knowles RR. J Am Chem Soc, 2020, 142: 5974–5979
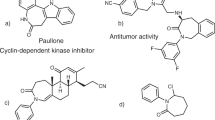
Bigge CF, Casimiro-Garcia A, Lee C, Risley HL, Schaum RP. Preparation of imidazo[4,5-b]pyridine compounds having both angiotensin II receptor antagonism and PPARγ activating activities. PCT Int Appl, 2008, WO 2008084303, 2008-07-17
Ito A, Sudo K, Imai E, Shimokawara T, Saishoji T, Mori M, Kimura R. Preparation of (heterocyclic methyl)azolylmethylcyclopentanol derivatives as agrochemicals and protective agents for industrial materials. PCT Int Appl, 2009, WO 2009088070, 2009-07-16
Rorufu UH, Geraruto AU. Preparation of (pyridylmethyl)tetralol derivatives as aromatase inhibitors. Jpn Kokai Tokkyo Koho, 1994, JP 06166674, 1994-06-14
Kawano E, Nagai M, Inubushi A, Shimada K, Tobari H. Nitrogen monoxide synthetase inhibitor comprising 2-aminopyridines as active ingredient. PCT Int Appl, 1997, WO 9709982, 1997-03-20
Eggler JF, Marfat A, Melvin LS Jr. Preparation of 6-aryloxy-3-arylmethylchromanones and -ols and analogs as lipoxygenase and LTD4 inhibitors. US Patent, 1991, US 5059609 A 1991-10-22
Eguchi Y, Chiba K, Goto M, Obaishi H, Kuboi Y. Preparation of cyclooctanone derivatives and cyclodecanone derivatives as cytokine production inhibitors. PCT Int Appl, 2002, WO 2002081420, 2002-10-17
Charton J, Girault-Mizzi S, Debreu-Fontaine MA, Foufelle F, Hainault I, Bizot-Espiard JG, Caignard DH, Sergheraert C. Bioorg Med Chem, 2006, 14: 4490–4518
Kong X, Vukomanovic D, Nakatsu K, Szarek WA. ChemMedChem, 2015, 10: 1435–1441
Katagiri Y, Takata H. Preparation of pyridine compound as harmful-arthropod control agent. PCT Int Appl, 2019, WO 2019083007, 2019-05-02
Kawada S, Matsumoto K, Arashima M, Takahashi T. Preparation of pyridine derivatives as thyroid hormone β receptor agonists. Jpn Kokai Tokkyo Hoho, 2012, JP 2012106996, 2012-06-07
Kawada S, Matsumoto K, Niijima M, Takahashi T. Preparation of 2-benzylpyridine and 2-phenoxypyridine derivatives as novel thyroid hormone β receptor agonists (thyromimetics). PCT Int Appl, 2010, WO 2010122980, 2010-10-28
Trost BM, Thaisrivongs DA, Hartwig J. J Am Chem Soc, 2011, 133: 12439–12441
Gentry EC, Knowles RR. Acc Chem Res, 2016, 49: 1546–1556
Li S, Zhu B, Lee R, Qiao B, Jiang Z. Org Chem Front, 2018, 5: 380–385
Dobmeier M, Herrmann JM, Lenoir D, König B. Beilstein J Org Chem, 2012, 8: 330–336
Elmore CS. Annu Rep Med Chem, 2009, 44: 515–534
Elmore CS, Bragg RA. Bioorg Med Chem Lett, 2015, 25: 167–171
Meanwell NA. J Med Chem, 2011, 54: 2529–2591
Alonso F, Beletskaya IP, Yus M. Chem Rev, 2002, 102: 4009–4092
Ranjan P, Pillitteri S, Van der Eycken EV, Sharma UK. Green Chem, 2020, 22: 7725–7736
The ee value of 44 was maintained after prolonging the reaction beyond completion. Moreover, under the established reaction conditions and in the presence of 100 equiv. of D2O, no deuteration product was observed by directly using 44 as the starting substrate. All results support the stability of the α-pyridyl stereocentre
Stern M, McNew JA. Mol Psychiatr, 2021, 26: 736–746
Dikalova AE, Pandey A, Xiao L, Arslanbaeva L, Sidorova T, Lopez MG, Billings Iv FT, Verdin E, Auwerx J, Harrison DG, Dikalov SI. Circ Res, 2020, 126: 439–452
Marchetto A, Ohmura S, Orth MF, Knott MML, Colombo MV, Arrigoni C, Bardinet V, Saucier D, Wehweck FS, Li J, Stein S, Gerke JS, Baldauf MC, Musa J, Dallmayer M, Romero-Pérez L, Hölting TLB, Amatruda JF, Cossarizza A, Henssen AG, Kirchner T, Moretti M, Cidre-Aranaz F, Sannino G, Grünewald TGP. Nat Commun, 2020, 11: 2423
The major diastereomer of these chiral products was used for evaluation
Motohashi H, Yamamoto M. Trends Mol Med, 2004, 10: 549–557
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21925103, 21901062), Key Technologies R&D Program of Henan (202102310005), Henan Normal University and Henan University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Supporting Information
11426_2021_1019_MOESM1_ESM.pdf
Divergent asymmetric synthesis of azaarene-functionalized cyclic alcohols through stereocontrolled Beckwith-Enholm cyclizations
Rights and permissions
About this article
Cite this article
Guo, Z., Chen, X., Fang, H. et al. Divergent asymmetric synthesis of azaarene-functionalized cyclic alcohols through stereocontrolled Beckwith-Enholm cyclizations. Sci. China Chem. 64, 1522–1529 (2021). https://doi.org/10.1007/s11426-021-1019-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1019-2