Abstract
The influence of tumor infiltrating lymphocytes on tumor growth and response to therapy is becoming increasingly apparent. While much work has focused on the role of T cell responses in anti-tumor immunity, the role of B cells in solid tumors is much less understood. Tumor infiltrating B cells have been found in a variety of solid tumors, including breast, ovarian, prostate, melanoma, and colorectal cancer. The function of B cells in solid tumors is controversial, with many studies reporting a pro-tumor effect, while other studies demonstrate a role for B cells in the anti-tumor immune response. In this review, we discuss the prognostic ability of B cells in solid tumors as well as the mechanisms by which B cells can either promote or suppress anti-tumor immunity. Additionally, we review current therapeutic strategies that may target both pro- and anti-tumor B cells.


Similar content being viewed by others
References
Naito Y et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–4.
Hu WH et al. Tumor-infiltrating CD8(+) T lymphocytes associated with clinical outcome in anal squamous cell carcinoma. J Surg Oncol. 2015;112(4):421–6.
Liu S et al. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48.
Kawai O et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113(6):1387–95.
Fukunaga A et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–31.
Oshikiri T et al. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol. 2003;84(4):224–8.
Davidsson S et al. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3(+) regulatory T cells with respect to lethal prostate cancer. Mod Pathol. 2012;26(3):448–55.
Huang Y et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget. 2015;6(19):17462–78.
Beyer M, Schultze JL. Regulatory T cells: major players in the tumor microenvironment. Curr Pharm Des. 2009;15(16):1879–92.
Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69(20):7895–8.
Erdag G et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–80.
Punt CJ et al. Anti-tumor antibody produced by human tumor-infiltrating and peripheral blood B lymphocytes. Cancer Immunol Immunother. 1994;38(4):225–32.
Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–82.
Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131(4):959–71.
Viau M, Zouali M. B-lymphocytes, innate immunity, and autoimmunity. Clin Immunol. 2005;114(1):17–26.
Liang Y et al. Toll-like receptor 2 induces mucosal homing receptor expression and IgA production by human B cells. Clin Immunol. 2011;138(1):33–40.
Jackson SM et al. Human B cell subsets. Adv Immunol. 2008;98:151–224.
Garraud O et al. Revisiting the B-cell compartment in mouse and humans: more than one B-cell subset exists in the marginal zone and beyond. BMC Immunol. 2012;13:63.
Dono M et al. The human marginal zone B cell. Ann N Y Acad Sci. 2003;987:117–24.
Zhang Y, Gallastegui N, Rosenblatt JD. Regulatory B cells in anti-tumor immunity. Int Immunol. 2015;27(10):521–30.
Wang WW et al. CD19 + CD24hiCD38hiBregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget. 2015;6(32):33486–99.
Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. 2016;4:40.
Zhou X et al. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol. 2016;53:27–35.
Zhou J et al. Enhanced frequency and potential mechanism of B regulatory cells in patients with lung cancer. J Transl Med. 2014;12:304.
Mohammed ZM et al. The relationship between lymphocyte subsets and clinico-pathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2013;109(6):1676–84.
Wei X et al. Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumour Biol. 2016;37(5):6581–8.
Blair PA et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–40.
Moir S et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205(8):1797–805.
Kardava L et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest. 2011;121(7):2614–24.
Titanji K et al. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. J Clin Invest. 2010;120(11):3878–90.
Saadoun D et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjogren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65(4):1085–96.
Martinez-Rodriguez M, Thompson AK, Monteagudo C. A significant percentage of CD20-positive TILs correlates with poor prognosis in patients with primary cutaneous malignant melanoma. Histopathology. 2014;65(5):726–8.
Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol. 2006;59(9):972–7.
Romaniuk A, Lyndin M. Immune microenvironment as a factor of breast cancer progression. Diagn Pathol. 2015;10(79). doi:10.1186/s13000-015-0316-y.
Thompson E et al. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol. 2016;29(3):249–58.
Banat GA et al. Immune and inflammatory cell composition of human lung cancer stroma. PLoS One. 2015;10(9):e0139073.
Del Mar Valenzuela-Membrives M et al. Progressive changes in composition of lymphocytes in lung tissues from patients with non-small-cell lung cancer. Oncotarget. 2016;7(44):71608–19.
Lundgren S et al. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J Ovarian Res. 2016;9:21.
Liao Y et al. Clinical implications of the tumor-infiltrating lymphocyte subsets in colorectal cancer. Med Oncol. 2013;30(4):727.
Woo JR et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014;12:30.
Lee HJ et al. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013;16(1):32–9.
Deschoolmeester V et al. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19.
Fortes C et al. Tumor-infiltrating lymphocytes predict cutaneous melanoma survival. Melanoma Res. 2015;25(4):306–11.
Tougeron D et al. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod Pathol. 2009;22(9):1186–95.
Brown JR et al. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2014;20(23):5995–6005.
Mahmoud SM et al. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2012;132(2):545–53.
Schmidt M et al. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin kappa C as a compatible prognostic marker in human solid tumors. Clin Cancer Res. 2012;18(9):2695–703.
Schmidt M et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68(13):5405–13.
Fan C et al. Building prognostic models for breast cancer patients using clinical variables and hundreds of gene expression signatures. BMC Med Genomics. 2011;4:3.
Alistar A et al. Dual roles for immune metagenes in breast cancer prognosis and therapy prediction. Genome Med. 2014;6(10):80.
Iglesia MD et al. Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin Cancer Res. 2014;20(14):3818–29.
Rody A et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011;13(5):R97.
Hanker LC et al. Prognostic evaluation of the B cell/IL-8 metagene in different intrinsic breast cancer subtypes. Breast Cancer Res Treat. 2013;137(2):407–16.
Ladanyi A et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother. 2011;60(12):1729–38.
Garg K et al. Tumor-associated B cells in cutaneous primary melanoma and improved clinical outcome. Hum Pathol. 2016;54:157–64.
Lardone RD et al. Cross-platform comparison of independent datasets identifies an immune signature associated with improved survival in metastatic melanoma. Oncotarget. 2016;7(12):14415–28.
Al-Shibli KI et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–7.
Fujimoto M et al. Stromal plasma cells expressing immunoglobulin G4 subclass in non-small cell lung cancer. Hum Pathol. 2013;44(8):1569–76.
Lohr M et al. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. 2013;333(2):222–8.
Al-Shibli K et al. The prognostic value of intraepithelial and stromal CD3-, CD117- and CD138-positive cells in non-small cell lung carcinoma. APMIS. 2010;118(5):371–82.
Santoiemma PP et al. Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol Oncol. 2016;143(1):120–7.
Nielsen JS et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18(12):3281–92.
Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res. 2016;22(12):3005–15.
Shimabukuro-Vornhagen A et al. Characterization of tumor-associated B-cell subsets in patients with colorectal cancer. Oncotarget. 2014;5(13):4651–64.
Berntsson J et al. Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int J Cancer. 2016;139(5):1129–39.
Meshcheryakova A et al. B cells and ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. PLoS One. 2014;9(6):e99008.
Parkes H et al. In situ hybridisation and S1 mapping show that the presence of infiltrating plasma cells is associated with poor prognosis in breast cancer. Br J Cancer. 1988;58(6):715–22.
Smorodin EP, Sergeyev BL. The level of IgG antibodies reactive to TF, Tn and alpha-Gal polyacrylamide-glycoconjugates in breast cancer patients: relation to survival. Exp Oncol. 2016;38(2):117–21.
Tomer Y, Sherer Y, Shoenfeld Y. Autoantibodies, autoimmunity and cancer (review). Oncol Rep. 1998;5(3):753–61.
Pectasides D et al. Clinical value of CA 15–3, mucin-like carcinoma-associated antigen, tumor polypeptide antigen, and carcinoembryonic antigen in monitoring early breast cancer patients. Am J Clin Oncol. 1996;19(5):459–64.
Kulic A et al. Anti-p53 antibodies in serum: relationship to tumor biology and prognosis of breast cancer patients. Med Oncol. 2009;27(3):887–93.
von Mensdorff-Pouilly S et al. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18(3):574–83.
Bosisio FM et al. Plasma cells in primary melanoma. Prognostic significance and possible role of IgA. Mod Pathol. 2016;29(4):347–58.
Karagiannis P et al. Elevated IgG4 in patient circulation is associated with the risk of disease progression in melanoma. Oncoimmunology. 2015;4(11):e1032492.
Hillen F et al. Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother. 2008;57(1):97–106.
Kurebayashi Y et al. Comprehensive immune profiling of lung adenocarcinomas reveals four immunosubtypes with plasma cell subtype a negative indicator. Cancer Immunol Res. 2016;4(3):234–47.
Dong HP et al. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol. 2006;125(3):451–8.
Yang C et al. Prognostic significance of B-cells and pSTAT3 in patients with ovarian cancer. PLoS One. 2013;8(1):e54029.
Song IH, et al. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast cancer. Cancer Res Treat. 2016. doi:10.4143/crt.2016.215.
Montfort A, et al. A strong B cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin Cancer Res. 2016;23(1):250–62.
Goc J et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74(3):705–15.
Germain C, Gnjatic S, Dieu-Nosjean MC. Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Front Immunol. 2015;6:67.
Cipponi A et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72(16):3997–4007.
de Chaisemartin L et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71(20):6391–9.
Sautes-Fridman C et al. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front Immunol. 2016;7:407.
Imahayashi S et al. Tumor-infiltrating B-cell-derived IgG recognizes tumor components in human lung cancer. Cancer Investig. 2000;18(6):530–6.
Yasuda M et al. Tumor-infiltrating B lymphocytes as a potential source of identifying tumor antigen in human lung cancer. Cancer Res. 2002;62(6):1751–6.
Mizukami M et al. Effect of IgG produced by tumor-infiltrating B lymphocytes on lung tumor growth. Anticancer Res. 2006;26(3A):1827–31.
Campa MJ et al. Interrogation of individual intratumoral B lymphocytes from lung cancer patients for molecular target discovery. Cancer Immunol Immunother. 2016;65(2):171–80.
Nzula S, Going JJ, Stott DI. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res. 2003;63(12):3275–80.
Hansen MH, Ostenstad B, Sioud M. Antigen-specific IgG antibodies in stage IV long-time survival breast cancer patients. Mol Med. 2001;7(4):230–9.
Coronella JA et al. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol. 2002;169(4):1829–36.
Pavoni E et al. Tumor-infiltrating B lymphocytes as an efficient source of highly specific immunoglobulins recognizing tumor cells. BMC Biotechnol. 2007;7:70.
Fremd C et al. Mucin 1-specific B cell immune responses and their impact on overall survival in breast cancer patients. Oncoimmunology. 2016;5(1):e1057387.
Wang Y et al. Focused antibody response in plasma cell-infiltrated non-medullary (NOS) breast cancers. Breast Cancer Res Treat. 2007;104(2):129–44.
Kotlan B et al. Immunoglobulin variable regions usage by B-lymphocytes infiltrating a human breast medullary carcinoma. Immunol Lett. 1999;65(3):143–51.
Coronella JA et al. Evidence for an antigen-driven humoral immune response in medullary ductal breast cancer. Cancer Res. 2001;61(21):7889–99.
Hansen MH, Nielsen H, Ditzel HJ. The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc Natl Acad Sci U S A. 2001;98(22):12659–64.
Pedersen AE et al. Wildtype p53-specific antibody and T-cell responses in cancer patients. J Immunother. 2011;34(9):629–40.
Maehara Y et al. Clinical implications of serum anti-p53 antibodies for patients with gastric carcinoma. Cancer. 1999;85(2):302–8.
Shiota G et al. Clinical significance of serum P53 antibody in patients with gastric cancer. Res Commun Mol Pathol Pharmacol. 1998;99(1):41–51.
Goodell V et al. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J Clin Oncol. 2006;24(5):762–8.
Kressner U et al. Increased serum p53 antibody levels indicate poor prognosis in patients with colorectal cancer. Br J Cancer. 1998;77(11):1848–51.
Peyrat JP et al. Prognostic significance of circulating P53 antibodies in patients undergoing surgery for locoregional breast cancer. Lancet. 1995;345(8950):621–2.
Mudenda B et al. The relationship between serum p53 autoantibodies and characteristics of human breast cancer. Br J Cancer. 1994;69(6):1115–9.
Crescioli S et al. IgG4 characteristics and functions in cancer immunity. Curr Allergy Asthma Rep. 2016;16(1):7.
Varga EM et al. Tolerant beekeepers display venom-specific functional IgG4 antibodies in the absence of specific IgE. J Allergy Clin Immunol. 2013;131(5):1419–21.
Chen LF et al. Elevated serum IgG4 defines specific clinical phenotype of rheumatoid arthritis. Mediat Inflamm. 2014;2014:635293.
Lin W et al. B cell subsets and dysfunction of regulatory B cells in IgG4-related diseases and primary Sjogren’s syndrome: the similarities and differences. Arthritis Res Ther. 2014;16(3):R118.
Karagiannis P et al. IgG4 antibodies and cancer-associated inflammation: insights into a novel mechanism of immune escape. Oncoimmunology. 2014;2(7):e24889.
Karagiannis P et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123(4):1457–74.
Raina A et al. Serum immunoglobulin G fraction 4 levels in pancreatic cancer: elevations not associated with autoimmune pancreatitis. Arch Pathol Lab Med. 2008;132(1):48–53.
Harshyne LA et al. Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro Oncol. 2015;18(2):206–15.
Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 Reaction. Int J Hepatol. 2014;2014:803876.
Kimura Y, Harada K, Nakanuma Y. Pathologic significance of immunoglobulin G4-positive plasma cells in extrahepatic cholangiocarcinoma. Hum Pathol. 2012;43(12):2149–56.
Tan J et al. Beneficial effect of T follicular helper cells on antibody class switching of B cells in prostate cancer. Oncol Rep. 2015;33(3):1512–8.
Fragoulis GE, Moutsopoulos HM. IgG4 syndrome: old disease, new perspective. J Rheumatol. 2010;37(7):1369–70.
Yanaba K et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–50.
Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176(2):705–10.
Flores-Borja F et al. CD19 + CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5(173):173ra23.
Carter NA et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186(10):5569–79.
Zhang Y et al. Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses. Int Immunol. 2016;28(9):423–33.
Zhang Y et al. B lymphocyte inhibition of anti-tumor response depends on expansion of Treg but is independent of B-cell IL-10 secretion. Cancer Immunol Immunother. 2013;62(1):87–99.
Iwata Y et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–41.
Kessel A et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 2012;11(9):670–7.
Tian J et al. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167(2):1081–9.
Parekh VV et al. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol. 2003;170(12):5897–911.
Tadmor T et al. The absence of B lymphocytes reduces the number and function of T-regulatory cells and enhances the anti-tumor response in a murine tumor model. Cancer Immunol Immunother. 2011;60(5):609–19.
Olkhanud PB et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71(10):3505–15.
Olkhanud PB et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69(14):5996–6004.
Ganti SN et al. Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth. Sci Rep. 2015;5:12255.
Bodogai M et al. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells. Cancer Res. 2015;75(17):3456–65.
Shao Y et al. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014;355(2):264–72.
Shalapour S et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521(7550):94–8.
de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–23.
Meyer S et al. A seven-marker signature and clinical outcome in malignant melanoma: a large-scale tissue-microarray study with two independent patient cohorts. PLoS One. 2012;7(6):e38222.
Staquicini FI et al. A subset of host B lymphocytes controls melanoma metastasis through a melanoma cell adhesion molecule/MUC18-dependent interaction: evidence from mice and humans. Cancer Res. 2008;68(20):8419–28.
Yang C et al. B cells promote tumor progression via STAT3 regulated-angiogenesis. PLoS One. 2013;8(5):e64159.
Xander P et al. Crosstalk between B16 melanoma cells and B-1 lymphocytes induces global changes in tumor cell gene expression. Immunobiology. 2013;218(10):1293–303.
Perez EC et al. B-1 lymphocytes increase metastatic behavior of melanoma cells through the extracellular signal-regulated kinase pathway. Cancer Sci. 2008;99(5):920–8.
Choi J et al. Differential expression of immune-related markers in breast cancer by molecular phenotypes. Breast Cancer Res Treat. 2012;137(2):417–29.
Rosser EC et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat Med. 2014;20(11):1334–9.
Yoshizaki A et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–8.
Matsumoto M et al. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity. 2011;34(5):703–14.
Bansal SC et al. Ex vivo removal of serum IgG in a patient with colon carcinoma: some biochemical, immunological and histological observations. Cancer. 1978;42(1):1–18.
Barbera-Guillem E et al. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48(10):541–9.
Kim S et al. B-cell depletion using an anti-CD20 antibody augments antitumor immune responses and immunotherapy in nonhematopoetic murine tumor models. J Immunother. 2008;31(5):446–57.
Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice. I. Immunity to a chemically induced tumor. J Immunol. 1978;121(1):359–62.
Monach PA, Schreiber H, Rowley DA. CD4+ and B lymphocytes in transplantation immunity. II. Augmented rejection of tumor allografts by mice lacking B cells. Transplantation. 1993;55(6):1356–61.
Qin Z et al. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4(5):627–30.
Manning TC et al. Antigen recognition and allogeneic tumor rejection in CD8+ TCR transgenic/RAG(−/−) mice. J Immunol. 1997;159(10):4665–75.
Aklilu M et al. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol. 2004;15(7):1109–14.
Velter C et al. Four cases of rituximab-associated melanoma. Melanoma Res. 2014;24(4):401–3.
Peuvrel L et al. Melanoma and rituximab: an incidental association? Dermatology. 2013;226(3):274–8.
Diehl L et al. The role of CD40 in peripheral T cell tolerance and immunity. J Mol Med (Berl). 2000;78(7):363–71.
Gladue RP et al. The CD40 agonist antibody CP-870,893 enhances dendritic cell and B-cell activity and promotes anti-tumor efficacy in SCID-hu mice. Cancer Immunol Immunother. 2011;60(7):1009–17.
Hunter TB et al. An agonist antibody specific for CD40 induces dendritic cell maturation and promotes autologous anti-tumour T-cell responses in an in vitro mixed autologous tumour cell/lymph node cell model. Scand J Immunol. 2007;65(5):479–86.
Carpenter EL et al. Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation. J Transl Med. 2009;7:93.
Ruter J et al. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther. 2010;10(10):983–93.
Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
Guan H et al. PD-L1 is a critical mediator of regulatory B cells and T cells in invasive breast cancer. Sci Rep. 2016;6:35651.
Guan H et al. PD-L1 mediated the differentiation of tumor-infiltrating CD19+ B lymphocytes and T cells in invasive breast cancer. Oncoimmunology. 2016;5(2):e1075112.
Colluru VT et al. Preclinical and clinical development of DNA vaccines for prostate cancer. Urol Oncol. 2013;34(4):193–204.
Ferraro B et al. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011;53(3):296–302.
Colluru VT, McNeel DG. B lymphocytes as direct antigen-presenting cells for anti-tumor DNA vaccines. Oncotarget. 2016;7(42):67901–18.
Wennhold K, et al. CD40-activated B cells induce anti-tumor immunity in vivo. Oncotarget. 2016.
Shin CA, et al. Co-expression of CD40L with CD70 or OX40L increases B-cell viability and antitumor efficacy. Oncotarget. 2016;7(29):46173–86.
Acknowledgements
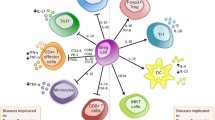
The authors would like to thank Jean-Paul Badjo for assisting with the artwork for Fig. 1 and Dr. Lynn Opdenaker for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Delaware INBRE program, with a grant from the National Institute of General Medical Sciences - NIGMS (P20 GM103446) from the National Institutes of Health and the state of Delaware.
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
This content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Rights and permissions
About this article
Cite this article
Flynn, N.J., Somasundaram, R., Arnold, K.M. et al. The Multifaceted Roles of B Cells in Solid Tumors: Emerging Treatment Opportunities. Targ Oncol 12, 139–152 (2017). https://doi.org/10.1007/s11523-017-0481-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-017-0481-x