Abstract
Purpose
Tumor segmentation constitutes a crucial step in simulating cancer growth and response to therapy. Incorporation of imaging data individualizes the simulation and assists clinical correlation with the predicted outcome. We adapted snakes to improve tumor segmentation including difficult cases with inherently inhomogeneous structure and poorly defined margins.
Methods
Snakes are flexible curves, based on the parameter-controlled deformation of an initial user-defined contour toward the boundary of the desired object, through the minimization of a suitable energy function. Although parameter-adjustment can yield fairly good results in homogeneous regions, traditional snakes often fail to provide an accurate segmentation result when both rigid and very elastic behavior is needed simultaneously to delineate the true outline of the tumor. We developed and tested a spatially adaptive active contour technique by introducing local snake bending, to improve traditional snakes performance for segmenting tumors. The key point of our method is the use of adaptable snake parameters, instead of constant ones, to adjust the bending of the curve according to the local edge characteristics. Our algorithm discriminates image regions according to underlying image features, such as gradient magnitude and corner strength. More specifically, it assigns each region a different “localized” set of parameters, one corresponding to a very flexible snake, and the other corresponding to a very rigid one, according to the local image characteristics.
Results
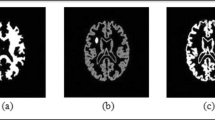
Qualitative results on more than 150 real MR images, as well as quantitative validation based on agreement with an expert clinician’s annotations of the true tumor boundaries, demonstrate our approach is highly efficient compared to traditional active contours and region growing. Due to the use of adaptable parameters in the snake evolution process, our approach outperforms the other two methods, and consistently follows an expert’s annotations. Statistical tests indicated significant difference between the results produced by our approach and two other algorithms traditional snakes and region growing, while multiple comparison showed that our method consistently outperformed those algorithms, with an average overlap of 89%, over the entire data set, while traditional snakes were at 82.5% and region growing at 59.2%. Furthermore, we performed several tests that demonstrate our method’s stability to different initial contours, as well as, to lower resolution images.
Conclusion
Our adaptive snake algorithm can spatially adapt to diverse image characteristics, producing outlines that mimic the true tumor boundaries. Results in MR datasets are very close to an expert clinician’s intuition about the tumor boundaries.
Similar content being viewed by others
References
Casciari J, Sotirchos S, Sutherland R (1992) Variations in tumor growth rates and metabolism with oxygen concentration, glucose concentration, and extracellular pH. J Cell Physiol 151: 386–394
Santini M, Rainaldi G (1999) Three-dimensional spheroid model in tumor biology. Pathobiology 67: 148–157
Wiarda D, Travis C (1997) Determinability of model parameters in a two-stage deterministic cancer model. Math Biosci 146: 1–13. doi:10.1016/S0025-5564(97)00025-4
Stamatakos G, Antipas V, Uzunoglu U (2006) A spatiotemporal, patient individualized simulation model of solid tumor response to chemotherapy in vivo: the paradigm of glioblastoma multi- forme treated by temozolomide. IEEE Trans Biomed Eng 53: 1467–1477
Adams R, Bischof L (1994) Seeded region growing. IEEE Trans Pattern Anal Mach Intell 16: 641–647
Beucher S (1991) The watershed transformation applied to image segmentation. In: Proceedings of scanning microscopy international, pp 299–314
Boykov Y, Jolly M (2001) Interactive graph cuts for optimal boundary and region segmentation of objects in ND images. In: International conference on computer vision, pp 105–112
Barrett W, Mortensen E (1997) Interactive live-wire boundary extraction. Med Image Anal 1: 331–341
Kass M, Witkin A, Terzopoulos D (1998) Snakes: active contour models. Int J Comput Vis 1: 321–331
Xu C, Prince J (1997) Gradient vector flow: a new external force for snakes. In: Proceedings of 1997 IEEE computer society conference on computer vision and pattern recognition, pp 66–71
Osher S, Sethian J (1988) Fronts propagating with curvature dependent speed: algorithms based on Hamilton-Jacobi formulations. J Comput Phys 79: 12–49
Caselles V, Kimmel R, Sapiro G (1997) Geodesic active contours. Int J Comput Vis 22: 61–79
Kim D, Park J (2004) Computer aided detection of kidney tumor on abdominal computed tomography scans. Acta Radiol 45: 791–795
Mancas M, Gosselin B (2003) Fuzzy tumor segmentation based on iterative watersheds. In: Proceedings of 14th ProRISC workshop circuits systems and signal process. Unpublished
Boykov Y, Jolly M (2000) Interactive organ segmentation using graph cuts. In: Medical image computing and computer-assisted intervention, pp 276–286
Boykov Y, Funka-Lea G (2006) Graph cuts and efficient nd image segmentation. Int J Comput Vis 70: 109–131
Esneault S, Hraiech N, Delabrousse E, Dillenseger J (2007) Graph cut liver segmentation for interstitial ultrasound therapy. Conf Proc IEEE Eng Med Biol Soc 1: 5247–5250
Cohen L, Cohen I, Ceremade P (1993) Finite-element methods for active contour models and balloons for 2-D and 3-D images. IEEE Trans Pattern Anal Mach Intell 15: 1131–1147
Nogueira S, Ruichek Y (2007) A new B-spline based active contour approach. In: IAPR conference on machine vision applications, pp 268–271
Lu R, Marziliano P, Thng C (2005) Liver tumor volume estimation bye semi-automatic segmentation method. In: Proceedings of 2005 IEEE engineering in medicine and biology 27th Annual Conference, pp 3296–3299
Cvancarova M, Albregtsen F, Brabrand K, Samset E (2005) Segmentation of ultrasound images of liver tumors applying snake algorithms and GVF. In: International congress of computer assisted radiology and surgery
Gu L, Xu J, Peters T (2006) Novel multistage three-dimensional medical image segmentation: methodology and validation. IEEE Trans Inf Technol Biomed 10: 740–748
Linguraru M et al (2009) Renal tumor quantification and classification in contrast-enhanced abdominal CT. Pattern Recognit 42: 1149–1161
Aleman-Flores M, Álvarez L, Caselles V (2007) Texture-oriented anisotropic filtering and geodesic active contours in breast tumor ultrasound segmentation. J Math Imaging Vis 28: 81–97
Kanakatte A, Gubbi J, Srinivasan B, Mani N, Kron T, Binns D, Palaniswami M (2008) Pulmonary tumor volume delineation in PET images using deformable models. Conf Proc IEEE Eng Med Biol Soc, pp 3118–3121
Farmaki C, Marias K, Sakkalis V, Graf N (2009) A spatially adaptive active contour method for improving semi-automatic medical image annotation. In: Proc World congress on medical physics and biomedical engineering, pp 1878–1881
Logan J (1997) Applied mathematics, chap 3, 2nd edn. Wiley, New york
Perona P, Malik J (1990) Scale-space and edge detection using anisotropic diffusion. IEEE Trans Pattern Anal Mach Intell 12: 629–639
Tsai D-M, Chao S-M (2005) An anisotropic diffusion-based defect detection for sputtered surfaces with inhomogeneous textures. Image Vis Comput 23: 325–338
Gonzalez RC, Woods RE (2002) Digital image processing. Prentice Hall, UK, pp, pp 134–137
Harris C, Stephens M (1988) A combined corner and edge detector. In: Alvey vision conference, pp 147–152
Chang F, Chen C, Lu C (2004) A linear-time component-labeling algorithm using contour tracing technique. Comput Vis Image Underst 93: 206–220
Zou K et al (2004) Statistical validation of image segmentation quality based on a spatial overlap index: scientific reports. Acad Radiol 11: 178–189
Daniel WW (1978) Applied nonparametric statistics. Houghton Mifflin, Boston
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farmaki, C., Marias, K., Sakkalis, V. et al. Spatially adaptive active contours: a semi-automatic tumor segmentation framework. Int J CARS 5, 369–384 (2010). https://doi.org/10.1007/s11548-010-0477-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-010-0477-9