Abstract
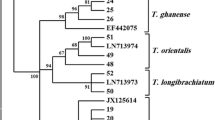
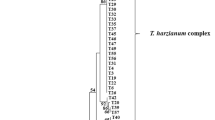
The biodiversity of Trichoderma was studied in the Northern half of the Nile valley in Egypt. 20 strains were isolated from 9 different geographic locations, representing 19 different habitats, all with a pH between 7.3 and 8.4. Only T. harzianum (three ITS1/2 haplotypes and three RAPD-genotypes) and the anamorph of Hypocrea orientalis were found. One of the T. harzianum haplotypes (4 strains) is new. The occurrence of T. harzianum haplotypes and of H. orientalis appeared to be essentially independent of the habitat (pH, plant, soil type), and also did not correlate with biochemical properties (cellulase and chitinase activity) of the individual strains. These two taxa seem to be indigenous to the Nile valley, their presence not being influenced by the agricultural history of the soils.
Similar content being viewed by others
Refferences
Caetan-Anolles G, Bassam B, Gresshoff PM (1992) Primer template interactions during DNA amplification fingerprinting with single arbitrary oligonucleotides. — Molecular and General Genetics 235: 121–127.
Chaverri P, Castlebury LA, Samuels GJ, Geiser DM (2003) Multilocus phylogenetic structure within the Trichoderma harzianum/Hypocrea lixii complex. — Molecular Phylogenetics and Evololution 27: 302–313.
Cumagun CJR, Hockenhull J, Lübeck M (2000): Characterization of Trichoderma isolates from Philippine rice fields by UP-PCR and rDNA-ITS1 analysis-identification of UP-PCR markers. — Journal of Phytopathology 148: 109–115.
Danielson R, Davey C (1973) The abundance of Trichoderma propagules and the distribution of species in forest soils. — Soil Biology and Biochemistry 5: 485–494.
Esposito E, Da Silva M (1998) Systematics and environmental application of the genus Trichoderma. — CRC Critical Reviews in Microbiology 24: 89–98.
Hamdan G (1961) Evaluation of Egyptian agriculture in Egypt. In: History of land use in arid regions, UNESCO, Paris: 119–142.
Hjeljord L, Tronsmo A (1998) Trichoderma and Gliocladium in biological control: an overview. In: Harman, G.E. and Kubicek, C.P., (eds.) Trichoderma and Gliocladium. Enzymes, biological control and commercial applications. pp. 131–151. Taylor and Francis Ltd. London, UK.
Jackson ML (1958) Soil chemical analysis. Constable and Co. London, UK
Johnson LF, Curl EA, Bond JH, Fribourg HA (1959) Methods for studying soil microflora-plant disease relationships. Burgess Publishing Co., Minneapolis, MN. 178 p.
Kimura M (1981) Estimation of evolutionary distances between homologous nucleotide sequences. — Proceedings of the National Academy of Sciences USA 78: 454–458.
Kindermann J, El-Ayouti Y, Samuels GJ, Kubicek CP (1998) Phylogeny of the genus Trichoderma based on sequence analysis of the internal transcribed spacer region 1 of the rDNA clade. — Fungal Genetics and Biology 24: 298–309.
Klein D, Eveleigh DE (1998) Ecology of Trichoderma. In: C.P. Kubicek. and G.E. Harman, (eds.) Trichoderma and GliocladiumBasic biology, taxonomy abnd genetics. pp. 57–73. Taylor & Francis Ltd. London, UK.
Kredics L, Antal ZS, Manczinger L, Szekeres A, Kevei F, Nagy E (2003) Influence of environmental parameters on Trichoderma strains with biocontrol potential. — Food Technology and Biotechnology 41: 37–42.
Kubicek CP, Penttilä ME (1998) Regulation of production of plant polysaccharide degrading enzymes by Trichoderma. In: Harman, G.E. and Kubicek, C.P. (eds.) Trichoderma and Gliocladium. Enzymes, biological control and commercial applications. pp. 49–71. Taylor & Francis Ltd. London, UK.
Kubicek CP, Mach RL, Peterbauer CK, Lorito M (2001) Trichoderma: from genes to biocontrol. — Journal of Plant Pathology 83: 11–23.
Kubicek CP, Bissett J, Druzhinina I, Kullnig-Gradinger C, Szakacs G (2003) Genetic and metabolic diversity of Trichoderma: a case study on South East Asian isolates. — Fungal Genetics and Biology 38: 310–319.
Kullnig CM, Szakacs G, Kubicek CP (2000) Molecular identification of Trichoderma species from Russia, Siberia and the Himalaya. — Mycological Research 104: 1117–1125.
Kullnig-Gradinger CM, Szakacs G, Kubicek CP (2002) Phylogeny and evolution of the fungal genus Trichoderma — a multigene approach. — Mycological Research 106: 757–767.
Lieckfeldt E, Kullnig CM, Kubicek CP, Samuels GJ, Börner T (2001) Trichoderma aureoviride: phylogenetic position and characterization. — Mycological Research 105: 313–322.
Lieckfeldt E, Samuels GJ, Börner T, Gams W (1998) Trichoderma koningii: neotypification and Hypocrea teleomorph. — Canadian Journal of Botany 76: 1507–1522.
Lopandic K, Prillinger H, Molnar O, Giménez-Jurado G (1996) Molecular characterization and genotypic identification of Metschnikowia species. — Systematic and Applied Microbiology 19: 393–402.
Mazen MB, Shaban GM (1983) Seasonal fluctuation of non-rhizosphere soil fungal in wheat field in Egypt. — Qtar University, Science Bulletin 3: 115–129.
Messner R, Schweigkofler W, Ibl M, Berg G, Prillinger H (1996) Molecular characterization of the plant pathogen Verticillium dahliae Kleb. using RAPD-PCR and sequencing of the 18S rRNA gene. — Journal of Phytopathology 144: 347–354.
Meyer W, Koch A, Niemann B, Epplen JT, Börner T (1991) Differentiation of species and strains among filamentous fungi by DNA fingerprinting. — Current Genetetics 19: 239–242.
Middendorf LR, Bruce JC, Bruce RC, Eckles RD, Grone DL, Roeme SC, Sloniker GD, Steffens DL, Sutter SL, Brumbaugh JA, Patonay G (1992) Continuous, on-line DNA sequencing using a versatile infrared laser scanner/electrophoresis apparatus. Electrophoresis 13, 487–494.
Moubasher AH, Abdel-Hafez SII (1987) Studies on the mycoflora of Egyptian soils. — Mycopathologia 63: 3–10.
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction nucleases. — Proceedings of the National Academy of Sciences USA 76: 5269–5273.
Nelson EE (1982) Occurrence of Trichoderma in a Douglas-fire soil. — Mycologia 74: 280–284.
Nicholas KB, Nicholas HB (1997) GeneDoc: a tool for editing multiple sequence alignments. Free software.
Norusis MJ (1993). SPSS for Windows, Professional Statistics, Release 6.0. SPSS Inc., Chicago
Papavizas GC, Lumsden RD (1982) Improved medium for isolation of Trichoderma spp. from soil. Plant Disease 66: 1019–1020.
Peterbauer CK, Heidenreich E, Baker RT, Kubicek CP (1992) Effect of benomyl and benomyl-resistance on cellulase formation by Trichoderma reesei and Trichoderma harzianum. — Canadian Journal of Microbiology 38: 1292–1297
Piper CG (1955): Soil and plant analysis. A laboratory manual of method, for the examination of soil and determination of the inorganic substituents of plants. International Publishers Inc. New York
Põldmaa K (2000) Generic delimitation of the fungicolous Hypocreaceae. — Studies in Mycology 45: 83–94.
Reissing JL, Strominger JL, Leloir LF (1955): A modified colorimetric method for the estimation of N-acetylamino sugars. — Journal of Biological Chemistry 217: 959–966.
Rifai MA (1969): A revision of the genus Trichoderma. — Mycological Papers 116: 1–56.
Said R (1993) The Revier Nile. Geology, hydrology and utilization. Pergamon Press
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: a Laboratory manual, 2nd. edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Samuels GJ (1996) Trichoderma: a review of biology and systematics of the genus. — Mycological Research 100: 923–935.
Samuels GJ, Petrini O, Kuhls K, Lieckfeldt E, Kubicek CP (1998) The Hypocrea schweinitzii complex and Trichoderma sect. Longibrachiatum. — Mycology 41: 1–54.
Shimodaira H, Hasegawa H (1999) Multiple comparisons of log-likelihoods with applications to phylogenetic interference. — Molecular Biology and Evolution 16: 1114–1116
Sivasithamparam K, Ghisalberti EL (1998) Secondary metabolism in Trichoderma and Gliocladium. In: Kubicek, C.P. and Harman, G.E. (eds.) Trichoderma and Gliocladium. Basic biology, taxonomy and genetics. pp. 139–191. Taylor & Francis Ltd. London, UK.
Somogi M (1953) A new reagent for the determination o sugars. — Journal of Biological Chemistry 160: 62–68.
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. — Nucleic Acids Research 24: 4876–4882.
Turner D, Kovacs W, Kuhls K, Lieckfeldt E, Peter B, Arisan-Atac I, Strauss J, Samuels GJ, Börner T, Kubicek CP (1997) Biogeography and phenotypic variation in Trichoderma sect. Longibrachiatum and associated Hypocrea species. — Mycological Research 101: 449–459.
Waterman MS (1986) Multiple sequence alignment by consensus. — Nucleic Acid Research 14: 9095–9102.
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand, DH, Sninsky JJ, White TJ (eds.) PCR protocols. A guide to methods and applications. Academic Press, San Diego, CA. pp. 315–322.
Widden P, Abitbol JJ (1980) Seasonality of Trichoderma species in a spruce-forest soil. — Mycologia 72: 775–784.
Wuczskowski M, Druzhinina I, Gherbawy Y, Klug B, Prillinger HJ, Kubicek CP (2003) Taxon pattern and genetic diversity of Trichoderma in a mid-European, primeval floodplain-forest. — Microbiological Research 158: 125–134.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gherbawy, Y., Druzhinina, I., Shaban, G.M. et al. Trichoderma populations from alkaline agricultural soil in the Nile valley, Egypt, consist of only two species. Mycol Progress 3, 211–218 (2004). https://doi.org/10.1007/s11557-006-0091-y
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11557-006-0091-y