Abstract
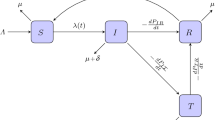
We study a mosquito-borne epidemic model where the vector population is distinct in aquatic and adult stages and a saturating effect of disease transmission is assumed to occur when the population of infectious carriers becomes large enough. A qualitative analysis, including centre manifold analysis, has been performed to determine the existence of stability–instability thresholds.
Similar content being viewed by others
References
Adongo, D., Fister, K.R., Gaff, H., Hartley, D.: Optimal control applied to Rift Valley fever. Nat. Res. Model. 26, 385–402 (2013)
Anderson, R.M., May, R.M.: Infectious Diseases in Humans: Dynamics and Control. Oxford University Press, Oxford (1991)
Anita, S., Capasso, V.: Stabilization of a reaction–diffusion system modelling malaria transmission. Discret. Contin. Dyn. Syst. B 17, 1673–1684 (2012)
Benedict, M.Q., Levine, R.S., Hawley, W.A., Lounibos, L.P.: Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 7, 76–85 (2007)
Buonomo, B., Vargas De-León, C.: Stability and bifurcation analysis of a vector-bias model of malaria transmission. Math. Biosci. 242, 59–67 (2013)
Cai, L., Guo, S., Li, X.Z., Ghosh, M.: Global dynamics of a dengue epidemic mathematical model. Chaos Solitons Fractals 42, 2297–2304 (2009)
Cai, L.M., Li, X.Z.: Global analysis of a vector–host epidemic model with nonlinear incidences. Appl. Math. Comput. 217, 3531–3541 (2010)
Capasso, V.: Mathematical Structures of Epidemic Systems. Lecture Notes in Biomathematics, vol. 97. Springer, Berlin (1993)
Capasso, V., Grosso, E., Serio, G.: I modelli matematici nella indagine epidemiologica. Applicazione all’epidemia di colera verificatasi in Bari nel 1973 (italian). Ann. Sclavo 19, 193–208 (1977)
Capasso, V., Serio, G.: A generalization of the Kermack–Mc Kendrick deterministic epidemic model. Math. Biosci. 42, 41–61 (1978)
Castillo-Chavez, C., Song, B.: Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 1, 361–404 (2004)
Corless, R.M.: Essential Maple 7: An Introduction for Scientific Programmers. Springer, New York (2002)
Diekmann, O., Heesterbeek, J.A.P.: Mathematical Epidemiology of Infectious Diseases. Model Building, Analysis and Interpretation. Wiley, Chichester (2000)
d’Onofrio, A.: Vaccination policies and nonlinear force of infection: generalization of an observation by Alexander and Moghadas (2004). Appl. Math. Comput. 168, 613–622 (2005)
Dumont, Y., Dufourd, C.: Spatio-temporal modeling of mosquito distribution. AIP Conf. Proc. 1404, 162–167 (2011)
Dushoff, J., Huang, W., Castillo-Chavez, C.: Backward bifurcations and catastrophe in simple models of fatal diseases. J. Math. Biol. 36, 227–248 (1998)
Esteva, L., Matias, M.: A model for vector transmitted diseases with saturation incidence. J. Biol. Syst. 9, 235–245 (2001)
Guckenheimer, J., Holmes, P.: Nonlinear Oscillations, Dynamical Systems and Bifurcations of Vector Fields. Springer, Berlin (1983)
Hethcote, H.W.: The mathematics of infectious diseases. SIAM Rev. 42, 599–653 (2000)
Jin, Y., Wang, W., Xiao, S.: An SIRS model with a nonlinear incidence rate. Chaos Solitons Fractals 34, 1482–1497 (2007)
Keeling, M.J., Rohani, P.: Modeling Infectious Diseases in Humans and Animals. Princeton University Press, Princeton (2008)
Lutambi, A.M., Penny, M.A., Smith, T., Chitnis, N.: Mathematical modelling of mosquito dispersal in a heterogeneous environment. Math. Biosci. 241, 198–216 (2013)
Maidana, N.A., Yang, H.M.: Describing the geographic spread of dengue disease by traveling waves. Math. Biosci. 215, 64–77 (2008)
Mecoli, M., De Angelis, V., Brailsford, S.C.: Using system dynamics to evaluate control strategies for mosquito-borne diseases spread by human travel. Comput. Oper. Res. 40, 2219–2228 (2013)
Moreno Glasser, C., de Castro Gomes, A.: Infestation of S. Paulo State, Brazil, by Aedes aegypti and Aedes albopictus (Portuguese). Rev. Saúde Pública 34, 570–577 (2000)
Moulay, D., Aziz-Alaoui, M.A., Cadivel, M.: The chikungunya disease: modeling, vector and transmission global dynamics. Math. Biosci. 229, 50–63 (2011)
Neteler, M., Roiz, D., Rocchini, D., Castellani, C., Rizzoli, A.: Terra and Aqua satellites track tiger mosquito invasion: modelling the potential distribution of Aedes albopictus in north-eastern Italy. Int. J. Health Geogr. 10, 49 (2011)
Ozair, M., Lashari, A.A., Jung, I.H., Okosun, K.O.: Stability analysis and optimal control of a vector-borne disease with nonlinear incidence. Discret. Dyn. Nat. Soc. Article ID 595487 (2012)
Poletti, P., Messeri, G., Ajelli, M., Vallorani, R., Rizzo, C., Merler, S.: Transmission potential of chikungunya virus and control measures: the case of Italy. PLoS One 3, e18860 (2011)
Rionero, S.: \(L^2\) stability of solutions to a nonlinear binary reaction–diffusion system of P.D.E.s. Rend. Mat. Acc. Lincei 16, 227–238 (2005)
Rionero, S.: A nonlinear \(L^2\) stability analysis for two species dynamics with dispersal. Math. Biosci. Eng. 3, 189–204 (2006)
Rionero, S.: A rigorous reduction of the \(L^2\)-stability of the solutions to a nonlinear binary reaction- diffusion system of P.D.E.s to the stability of the solutions to a linear binary system of ODEs. J. Math. Anal. Appl. 319, 377–397 (2006)
Rionero, S.: On the nonlinear stability of the critical points of an epidemic SEIR model via a novel Lyapunov function. Rend. Acc. Sci. Fis. Nat. Napoli 75, 115–129 (2008)
Rionero, S.: Stability of ternary reaction-diffusion dynamical systems. Rend. Lincei Mat. Appl. 22, 245–268 (2011)
Rionero, S.: \(L^2\)-energy decay of convective nonlinear PDEs reaction–diffusion systems via auxiliary ODEs systems. Ric. Mat. (2015). doi:10.1007/s11587-015-0231-2
Rodrigues, H.S., Monteiro, M.T.T., Torres, D.F.M.: Vaccination models and optimal control strategies to dengue. Math. Biosci. 247, 1–12 (2014)
Takahashi, L.T., Maidana, N.A., Ferreira, W.C., Jr, Pulino P., Yang, H.M.: Mathematical models for the Aedes aegypti dispersal dynamics: travelling waves by wing and wind. Bull. Math. Biol. 67, 509–528 (2005)
Tolle, M.A.: Mosquito-borne diseases. Curr. Probl. Pediatr. Adolesc. Health Care 39, 97–140 (2009)
van den Driessche, P., Watmough, J.: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180, 29–48 (2002)
Xiao, D., Ruan, S.: Global analysis of an epidemic model with nonmonotone incidence rate. Math. Biosci. 208, 419–429 (2007)
Acknowledgments
The work of E. A.-V. has been partially supported by SNI, under Grant 15284. The work of B. B. has been partially supported by local Grant 2015-2016 ‘Analysis of Complex Biological Systems’ and has been performed under the auspices of the Italian National Group for the Mathematical Physics (GNFM) of National Institute for Advanced Mathematics (INdAM). The authors gratefully acknowledge Noé Chan-Chi and Erika Rivero-Esquivel for their helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Salvatore Rionero.
Appendix: Proof of Routh–Hurwitz conditions in Theorem 4
Appendix: Proof of Routh–Hurwitz conditions in Theorem 4
Consider the cubic polynomial \(P( \lambda ) = \lambda ^3 + a_2 \lambda ^2 +a_1 \lambda + a_0\) in (20), where the coefficients are given in (21). We first show that \(a_0\) is positive.
Recall that
Substituting \(H^{*}\) given in (19), we have that
where the last equality follows from (18). In view of (24), we get
which is positive.
Similarly, using (24) for \(a_1\), it follows
so that \(a_1\) is positive.
Finally, from (25), (26) and \(a_2\) in (21) it is easy to show that \(a_2a_1-a_0>0\), so that all the Routh–Hurwitz conditions in Theorem 4 are verified.
Rights and permissions
About this article
Cite this article
Avila-Vales, E., Buonomo, B. Analysis of a mosquito-borne disease transmission model with vector stages and nonlinear forces of infection. Ricerche mat. 64, 377–390 (2015). https://doi.org/10.1007/s11587-015-0245-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11587-015-0245-9