Abstract
Objective
To investigate the effects of Chlorophytum borivilianum (CB) on sexual dysfunction, loss of body weight, and lack of libido in hyperglycemic rats induced with streptozotocin or alloxan.
Methods
Wistar strain male albino rats were divided into five groups of six animals each: the control group (2% polyvinylpyrollidone solution), the streptozotocin control group (50 mg/kg), the alloxan control group (100 mg/kg), the streptozotocin + CB treated group (200 mg/kg), and the alloxan + CB treated group (200 mg/kg). Only after confirming the induction of diabetes, the animals of test groups were treated with CB. The sexual behavior of male rats of in presence of female rat in a special cage was recorded. The effects of induced diabetes in control groups and on simultaneous extract treatment in CB treated groups were tested for sexual parameters. The parameters evaluated included mount, ejaculation, and intromission latencies/frequencies, hesitation time, and penile erection index. Parallel to this, using a separate set of similarly treated animals, the influence of diabetes and CB treatment on anabolism and weight of secondary sexual organs were determined on day 0 and day 28 of the treatment.
Results
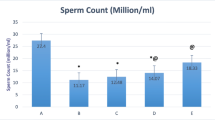
CB extract treatment ameliorated the diabetes-induced dysfunction at 200 mg/kg dose. There was very low weight loss (P<0.05) in CB-treated animals as compared to the diabetic control. There was a very high latency time (P<0.05) in the diabetic animals, whereas the latency time was very low in CB-treated animals. Mount, intromission, and ejaculation frequencies were very high (P<0.01) in CB-treated animals, while streptozotocin and alloxan groups animals had a very significantly lower sexual behavior (P<0.05) compared to the normo-glycemic control group animals.
Conclusion
CB can significantly ameliorate diabetes-induced sexual dysfunction. Polysaccharide and saponin-rich aqueous extract appears to have the most suitable effects on diabetes and its associated effects on sexual functionality.
Similar content being viewed by others
References
Shah J. Erectile dysfunction through the ages. BJU Int 2002;90:433–441.
Shabsigh RM, Shah M, Sand M. Erectile dysfunction and men’s health: developing a comorbidity risk calculator. J Sex Med 2008;5:1237–1243.
Suter S. Impotence: evaluation and treatment in general practice—what is reliable? Ther Umsch 1998;55:357–360.
Derogatis LR. Clinical and research evaluations of sexual dysfunctions. Adv Psychosom Med 2008;29:7–22.
Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Fernando DJ, Levy JC. Erectile dysfunction among men with diabetes is strongly associated with premature ejaculation and reduced libido. J Sex Med 2008;5:2125–2134.
Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Klinefelter GR, Kempinas WG. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl 2006;29:482–488.
Aybek H, Aybek Z, Rota S, Sen N, Akbulut M. The effects of diabetes mellitus, age, and vitamin E on testicular oxidative stress. Fertil Steril 2008;90:755–760.
Puri HS, ed. Rasayana—ayurvedic herbs for longevity and rejuvenation. London: Taylor and Francis; 2003:212–224.
Triveni A, ed. Rasendrasarasangrah: Vajikaranadhikar. Rajkot: Nutan Press; 1975:617–643.
Mishra SN, ed. Bhaisajya ratnavali. 1st ed. Varanasi: Chaukambha Surbharti Prakashan; 2005:1008–133.
Thakur M, Bhargava S, Dixit VK. Immunomodulatory activity of Chlorophytum borivilianum Sant F. Evid Based Complement Altern Med 2007;4:419–423.
Thakur M, Dixit VK. Effect of Chlorophytum borivilianum on androgenic and sexual behavior in male rats. Indian Drugs 2006;43:300–306.
Narasimhan S, Govindarajan R, Madhavan V, Thakur M, Dixit VK, Mehrotra S, et al. Action of (2 1) fructooligopolysaccharide fraction of Chlorophytum borivilianum against streptozotocin-induced oxidative stress. Planta Med 2006;72:1421–1424.
Diamond MP, Moley KH, Pellicer A, Vaughn W, DeCherney AH. Effects of streptozotocin- and alloxan-induced diabets mellitus on mouse follicular and early embryo development. J Reprod Fertil 1989;86:1–10.
Ottoni EB. EthoLog 2.2: a tool for the transcription and timing of behavior observation sessions. Behav Res Methods Instrum Comput 2000;32:446–449.
Govindrajan R, Sreevidya N, Vijaykumar M, Thakur M, Dixit VK, Mehrotra S, et al. In vitro antioxidant activity of ethanolic extract of Chlorophytum borivilianum. Nat Prod Sci 2005;11:165–169.
Saxena S, Dixit VK. Role of total alkaloids of Mucuna pruriens Baker. in spermatogenesis in male rats. Indian J Nat Prod 1987;3:3–7.
Malmnas CO, Meyerson BJ. p-chlorophenylalanine and copulatory behaviour in male rat. Nature 1971;232:398–400.
Ang HH, Ngai TH. Aphrodisiac evaluation in non-copulator male rats after chronic administration of Eurycoma longifolia Jack. Fundam Clin Pharmacol 2001;15:265–268.
Agmo A. Male rat sexual behavior. Brain Res Protoc 1997;1:203–209.
Benassi-Benelli A, Ferrari F, Quarantotti BP. Penile erection induced by apomorphine and N-n-apopolymorphine in rats. Arch Int Pharmacodyn Ther 1979;242:241–247.
Islam MW, Tariq M, Ageel AM, al-Said MS, al-Yhya AM. Effect of Salvia hematodes on sexual behavior of male rats. J Ethnopharmacol 1991;33:67–72.
Benelli A, Arletti R, Poggioli R, Cavazutti E, Mameli M, Bertolini A. Effect of potassium channel modulator on male sexual behaviors. Life Sci 1997;60:263–267.
Ejskjaer N, Christiansen JS. Sexual dysfunction in diabetes mellitus. Ugeskr Laeger 2002;164:4789–4791.
Steger RW. Testosterone replacement fails to reverse the adverse of streptozotocin-induced diabetes on sexual behavior in the male rat. Pharmacol Biochem Behav 1990;35:577–582.
Kniel PC, Junker U, Perrin IV, Bestetti GE, Rossi GL. Varied effects of experimental diabetes on the autonomic nervous system of the rat. Lab Invest 1986;54:523–530.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, M., Bhargava, S., Praznik, W. et al. Effect of Chlorophytum Borivilianum Santapau and Fernandes on sexual dysfunction in hyperglycemic male rats. Chin. J. Integr. Med. 15, 448–453 (2009). https://doi.org/10.1007/s11655-009-0448-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-009-0448-6