Abstract
Objective
To establish a novel cardiocentesis method for withdrawing venous blood from the right atrium, and to improve an acute blood stasis rat model using an ice bath and epinephrine hydrochloride (Epi) while considering the 3Rs (reduction, refinement, and replacement) of humane animal experimentation.
Methods
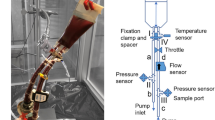
An acute blood stasis model was established in male Sprague-Dawley rats by subcutaneous injection (s.c.) Epi (1.2 mg/kg) administration at 0 h, followed by a 5-min exposure to an ice-bath at 2 h and s.c. Epi administration at 4 h. Control rats received physiological saline. Rats were fasted overnight and treated with Angelicae Sinensis Lateralis Radix (ASLR) and Pheretima the following day. Venous blood was collected using our novel cardiocentesis method and used to test whole blood viscosity (WBV), prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen (FIB) content.
Results
The rats survived the novel cardiocentesis technique; WBV value returned to normal while hematological parameters such as hemoglobin level and red blood cell count were restored to >94% of the corresponding values in normal rats following a 14-day recovery. Epi (1.2 mg/kg, s.c.) combined with a 5-min exposure to the ice bath replicated the acute blood stasis rat model and was associated with the highest WBV value. In rats showing acute blood stasis, ASLR treatment [4 g/(kg·d) for 8 days] decreased WBV by 9.98%, 11.09%, 9.34%, 9.00%, 7.66%, and 7.03% (P<0.05), while Pheretima treatment [2.6 g/(kg·d), for 8 days] decreased WBV by 25.49%, 25.94%, 16.28%, 17.76%, 11.07%, and 7.89% (P<0.01) at shear rates of 1, 3, 10, 30, 100, and 180 s−1, respectively. Furthermore, Pheretima treatment increased APTT significantly (P<0.01).
Conclusions
We presented a stable, reproducible, and improved acute blood stasis rat model, which could be applied to screen drugs for promoting blood circulation and eliminating blood stasis.
Similar content being viewed by others
References
Chen KJ. Blood stasis syndrome and its treatment with activating blood circulation to remove blood stasis therapy. Chin J Integr Med 2012;18:891–896.
Gong MF, Li FX, Duan SL. Analysis of blood stasis patient’s hemorheology and thrombosis in vitro. J Hubei Univ Chin Med (Chin) 2000;2:25–26.
Mao TM, Lin JH. Preliminary survey of an experimental model on “blood stagnation”. J Beijing Med Univ (Chin) 1985;17:246–248.
Li WX, Huang MY, Tang YP, Guo JM, Shang EX, Liu X, et al. Establishment and optimization of acute blood stasis rat model. Chin Pharmacol Bull (Chin) 2011;27:1761–1765.
Liu L, Duan JA, Tang YP, Guo JM, Yang NY, Ma HY, et al. Taoren-Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation. J Ethnopharmacol 2012;139:381–387.
Li WX, Tang YP, Guo JM, Huang MY, Qian DW, Duan JA. Comparative assessing the effects of Angelica root and Chuangxiong on the hemorheology and blood coagulation function in acute blood stasis rats. Chin J Integr Tradit West Med (Chin) 2012;32:806–811.
Li HX, Han SY, Wang XW, Ma X, Zhang K, Wang L, et al. Effect of the carthamins yellow from Carthamus tinctorius L. on hemorheological disorders of blood stasis in rats. Food Chem Toxicol 2009;47:1797–1802.
Blumgart HL, Weiss S. Clinical studies on the velocity of blood flow: IX. The pulmonary circulation time, the velocity of venous blood flow to the heart and related aspects of the circulation in patients with cardiovascular disease. J Clin Invest 1928;5:343–377.
Joslin JO. Blood collection techniques in exotic small mammals. J Exot Pet Med 2009;18:117–139.
Wei FH, Mo ZX, Wang ZG. The influence of irrigation stimulability on the independent activity of the mices. Chin Pharmacol Bull (Chin) 2004;3:356–357.
Zhao LM, Huang YY, Zheng DX, Wu AQ, Han J. Study on the impact of different ways in giving toxicant to the rats. J China Tropi Med (Chin) 2004;4:202–203.
Gao XY, Hu YY, Hu WP, Zhou CS. Direction of clinical research on determination of whole blood viscosity and hyperviscosity syndrome. Proceedings of international conference of blood stasis syndrome and the research of promoting blood circulation to remove blood stasis; 2007 Aug 25–27; Harin, China. 2007:119–120.
Richards RS, Nwose EU. Blood viscosity at different stages of diabetes pathogenesis. Br J Biomed Sci 2010;67:67–70.
Devereux RB, Drayer JI, Chien S, Pickering TG, Letcher RL, DeYoung JL, et al. Whole blood viscosity as a determinant of cardiac hypertrophy in systemic hypertension. Am J Cardiol 1984;54:592–595.
Gyawali P, Richards RS, Nwose EU, Bwititi PT. Whole-blood viscosity and metabolic syndrome. Clin Lipidol 2012;7:709–719.
Kowal P, Marcinknowska-Gapinska A, Elikowski W, Chalupka Z. Comparison of whole blood viscosity in vascular diseases. Pol Merkur Lekarski 2003;15:515–517.
De Backer TL, De Buyzere M, Segers P, Carlier S, De Sutter J, Van de Wiele C. The role of whole blood viscosity in premature coronary artery disease in women. Atherosclerosis 2002;165:367–373.
Antonova N, Velcheva I. Hemorheological disturbances and characteristic parameters in patients with cerebrovascular disease. Clin Hemorheol Microcirc 1999;21:405–408.
Ciuffetti G, Schillaci G, Lombardini R, Pirro G, Vaudo G, Mannarino E. Prognostic impact of low-shear whole blood viscosity in hypertensive men. Eur J Clin Invest 2005;35:93–98.
Kim W, Park SK, Kang KP, Lee DH, Kim SY, Jung JM, et al. Changes in whole blood viscosity at low shear rates correlate with intravascular volume changes during hemodialysis. Int J Artif Organs 2012;35:425–434.
Lee SR, Jung JM, Jung LY, Lee JH, Lee SH, Rhee KS, et al. Elevated coronary whole blood viscosity in acute coronary syndrome patients. Clin Hemorheol Microcirc 2013;55:85–94.
Baskurt OK, Boynard M, Cokelet GC, Connes P, Cooke BM, Forconi S, et al. New guidelines for hemorheological laboratory techniques. Clin Hemorheol Microcirc 2009;42:75–97.
Cui EX, Long LH, Zhang GQ, Cao YX, Xie EY, Guo ZJ. Analgesic effect and promoting blood circulation and eliminating blood stasis effect of Compound Huanglu Capsule. Chin Med Mat (Chin) 2009;32:591–595
Zhao XJ, Zhang Y, Meng XL, Yin PY, Deng C, Chen J, et al. Effect of a traditional Chinese medicine preparation Xindi soft capsule on rat model of acute blood stasis: a urinary metabonomics study based on liquid chromatography mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2008;873:151–158.
He L, Jiang WY, Mao TM. Preliminary exploration on establishing a simulated model of acute and chronic afterqi-stagnation blood stasis by adrenaline injection. Chin J Integr Tradit West Med (Chin) 2004;244-246.
Xu LF. Effect of storage temperature on epinephrine hydrochloride injection titer. Chin Pharm J (Chin) 1978;2:67.
Guan HY, Lon J, Hu DQ, Xu J, Lu LC. Study on influence of irradiation on content of epinephrine hydrochloride injection. J China Pharm (Chin) 2013;22:35–37.
Jin W, Peng XS, Jiang WM, Yang YJ. Determination of S-isomer in epinephrine hydrochloride injection by capillary electrophoresis and stability evaluation. Chin J Pharm Anal (Chin) 2012;32:273–276.
Author information
Authors and Affiliations
Contributions
Huang S performed the experiments and wrote the article. Xu F designed the experiments and revised the article. Wang CQ performed experiments and revised the article. Wang YY, Shang MY, and Wang X revised the article. Cai SQ guided the design of experiments and article writing.
Corresponding author
Ethics declarations
The authors declare no competing financial interest.
Additional information
Supported by the National Science and Technology Major Project for Major New Drugs Innovation and Development of China (No. 2009ZX09502-023-4), and National Technology Support Program (No. 2006BAI09B06)
Rights and permissions
About this article
Cite this article
Huang, S., Xu, F., Wang, Yy. et al. Improvement and Application of Acute Blood Stasis Rat Model Aligned with the 3Rs (Reduction, Refinement and Replacement) of Humane Animal Experimentation. Chin. J. Integr. Med. 26, 292–298 (2020). https://doi.org/10.1007/s11655-014-2008-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-014-2008-y