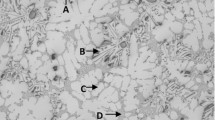
The effect of combinations of several deoxidizers, i.e., Mg-Al, Mg-Ti, Al-Ti, and Ce-Al, on the solidification structure of Fe-2 mass pct Ni-1 mass pct Mn-1 mass pct Mo alloy melt was investigated using a melt sampling and quenching method. Using this method, we evaluated the catalytic potency of several complex inclusion particles by taking the inclusion evolution process into account. Fine equiaxed crystals were obtained in the Mg-Ti-deoxidized steel wherein the MgO(MgAl2O4)-TiN complex compounds formed. However, the longer the holding time at high temperatures, the larger the fraction of Ti2O3, and very fine TiN formed because of microsegregation during solidification, resulting in poor equiaxed crystals. When the steel was deoxidized with Mg-Al, the initial structure was dominantly columnar. However, the longer the holding time, the larger the fraction of MgAl2O4 spinel, resulting in the formation of fine equiaxed crystals. Ce-Al complex deoxidation provided a relatively small portion of equiaxed crystals, whereas Ti-Al deoxidation produced the fewest equiaxed crystals because of the formation of alumina. The effectiveness of each inoculant particle for the crystallization of the primary δ-iron was explained well by the lattice disregistry concept.













Similar content being viewed by others
References
Ø. Grong: Metallurgical Modeling of Welding, 2nd edition, Institute of Materials, London, 1997, pp. 221-300.
Ø. Grong and D.K. Matlock: Int. Met. Rev., 1986, vol. 31, pp. 27-48.
O.M. Akselsen, Ø. Grong, and P.E. Kvaale: Metall. Mater. Trans. A, 1986, vol. 17A, pp. 1529-36.
J. Takamura and S. Mizuguchi: Proc. 6th Int. Iron Steel Cong., vol. 3, ISIJ, Tokyo, 1990, pp. 591-97.
E.A. Metzbower, H.K.D.H. Bhadeshia, and R.H. Phillips: Mater. Sci. Technol., 1994, vol. 10, pp. 56-59.
Z. Yang and T. Debroy: Metall. Mater. Trans. B, 1999, vol. 30B, pp. 483-93.
T. Koseki and G. Thewlis: Mater. Sci. Technol., 2005, vol. 21, pp. 867-79.
G. Thewlis: Mater. Sci. Technol., 2006, vol. 22, pp. 153-66.
K.S. Bang, C. Park, and S. Liu: J. Mater. Sci., 2006, vol. 41, pp. 5994-6000.
T. Koseki, H. Kato, M. Tsutsumi, K. Kasaki, and J. Inoue: Int. J. Mater. Res., 2008, vol. 99, pp. 347-51.
H.K. Sung, S.Y. Shin, W. Cha, K. Oh, S. Lee, and N.J. Kim: Mater. Sci. Eng. A, 2011, vol. 528, pp. 3350-57.
H. Suito, A.V. Karasev, M. Hamada, and R. Inoue, and K. Nakajima: ISIJ Int., 2011, vol. 51, pp. 1151-62.
J.L. Caron, S.S. Babu, and J.C. Lippold: Metall. Mater. Trans. A, 2011, vol. 42, pp. 4015-31.
A.O. Kluken, Ø. Grong and G. Rørvik: Metall. Trans. A., 1990, vol. 21A, pp. 2047-58.
H. Terasaki and Y. Komizo: Sci. Technol. Weld. Join., 2006, vol. 11, pp. 561-66.
T. Yamada, H. Terasaki and Y. Komizo: Sci. Technol. Weld. Join., 2008, vol. 13, pp. 118-25.
T. Koseki, S. Ohkita and N. Yurioka: Sci. Technol. Weld. Join., 1997, vol. 2, pp. 65-9.
Y. Ito and M. Nakanishi: Sumitomo Search, 1976, vol. 15, pp. 42-62.
N. Mori, H.Homma, M. Wakabayshi and S. Ohkita: J. Jpn. Weld. Soc., 1981, vol. 50, pp. 786-93.
T. Nishizawa: ISIJ Int., 2000, vol. 40, pp. 1269-74.
B.L. Bramfitt: Metall. Trans., 1970, vol. 1, pp. 1987-95.
T. Ohashi, T. Hiromoto, H. Fujii, Y. Nuri and K. Asano: Tetsu-to-Hagané, 1976, vol. 62, pp. 614-23.
K. Nakajima, H. Hasegawa, S. Khumkoa and S. Mizoguchi: Metall. Mater. Trans. B., 2003, vol. 34B, pp. 539-47.
K. Nakajima, H. Ohta, H. Suito and P. Jönsson: ISIJ Int., 2006, vol. 46, pp. 807-13.
K. Sakata and H. Suito: Metall. Mater. Trans. B., 1999, vol. 30B, pp. 1053-63.
M. Guo and H. Suito: ISIJ Int., 1999, vol. 39B, pp. 722-29.
G.V. Pervushin and H. Suito: ISIJ Int., 2001, vol. 41, pp. 728-37.
H. Ohta and H. Suito: ISIJ Int., 2006, vol. 46, pp. 22-28.
J.H. Park: CALPHAD, 2011, vol. 35, pp. 455-62.
T. Koseki, H. Inoue, Y. Fukuda and A. Nogami: Sci. Technol. Adv. Mater., 2003, vol. 4, pp. 183-95.
S. Mridha, D.H. Jack: Metallography, vol. 15, 1982, pp. 163-75.
J.H. Park, D.J. Kim, and D.J. Min: Metall. Mater. Trans. A, 2012, vol. 43A, pp. 2316-24.
M. Hino and K. Ito: Thermodynamic Data for Steelmaking, Tohoku University Press, Sendai, 2010, pp. 10-34.
J.H. Park and H. Todoroki: ISIJ Int., 2010, vol. 50, pp. 1333-46.
D.R. Poirier and G.H. Geiger: Transport Phenomena in Materials Processing, TMS, Warrendale, PA, 1994, pp. 62-71.
www.factsage.com (accessed October 2011).
C.W. Bale, E. Belisle, P. Chartrand, S.A. Decterov, G. Eriksson, K. Hack, I.H. Jung, Y.B. Kang, J. Melancon, A.D. Pelton, C. Robelin, and S. Petersen: CALPHAD, 2009, vol. 33, pp. 295-311.
J.H. Park and Y.B. Kang: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 791-98.
J.H. Park, S.B. Lee and H.R. Gaye: Metall. Mater. Trans. B, 2008, vol. 39, pp. 853-61.
G.V. Pervushin and H. Suito: ISIJ Int., 2001, vol. 41, pp. 748-56.
J.H. Park, J.S. Park, Y. Huh, and C.H. Lee: unpublished research.
T. Koseki and H. Inoue: J. Jpn Inst. Met., 2001, vol. 65, pp. 644-51.
T. Koseki: Bull. Iron Steel Inst. Japan, 2010, vol. 15, pp. 30-35.
H. Fujimura, S. Tsuge, Y. Komizo and T. Nishizawa: Tetsu-to-Hagané, 2001, vol. 87, pp. 29-34.
K. Isobe: ISIJ Int., 2010, vol. 50, pp. 1972-80.
M. Wako and N. Sano: ISIJ Int., 2007, vol. 47, pp. 627-32.
G. Fiquet, P. Richet and G. Montagnac: Phys. Chem. Minerals, 1999, vol. 27, pp. 103-11.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted: December 1, 2011.
Appendix
Appendix
To illustrate the calculation of disregistry, we provide examples for several oxide inclusions nucleating TiN.[21] In these examples, the MgAl2O4 spinel, Al2O3, MgO, and Ti2O3 were selected as nucleation sites for TiN. The crystallographic relationships of each case are illustrated in Figures A1 and A2, and the corresponding parameters for Eq. [4] are listed in Table IV.
Case I; MgAl2O4(100)//TiN(100)
As shown in Figure A1(a), the (100) plane of TiN is superimposed on the (100) plane of spinel. The three lowest-index directions of TiN and MgAl2O4 are \( \left[ {\overline{1} 00} \right] \), \( \left[ {\overline{1} 10} \right] \) and \( \left[ {010} \right] \). The distances along these directions are given in Table IV. In accordance with Eq. [4], the disregistry equation for Case 1 was written as follows:
Case II; MgO(100)//TiN(100)
As shown in Figure A1(b), the (100) plane of TiN is superimposed on the (100) plane of MgO. Using a similar analysis to that used in Case I, Eq. [4] was written as follows:
Case III; Ti2O3(0001)//TiN(100)
Because the lattice disregistry is 23.46 pct for the (110) plane of TiN and 48.36 pct for the (111) plane of TiN, the (100) of TiN is superimposed on the (0001) plane of Ti2O3, as shown in Figure A2(a). Equation [4] was therefore written as:
Case IV; Al2O3(0001)//TiN(100)
Because the lattice disregistry of the (110) plane of TiN is 23.7 pct and that of the (111) plane of TiN is 51.7 pct, the (100) plane of TiN is superimposed on the (0001) of Al2O3, as shown in Figure A2(b). Using a similar analysis as in Case III yielded the following Eq. [4]:
Rights and permissions
About this article
Cite this article
Park, J.S., Lee, C. & Park, J.H. Effect of Complex Inclusion Particles on the Solidification Structure of Fe-Ni-Mn-Mo Alloy. Metall Mater Trans B 43, 1550–1564 (2012). https://doi.org/10.1007/s11663-012-9734-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9734-3