Abstract
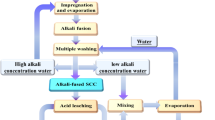
A three-step process was employed to separate cryolite from used carbon cathodes, known as spent pot lining (SPL), and obtain valuable carbon. The process comprised leaching of NaF from the imbedded electrolyte with water, followed by leaching of Na3AlF6, CaF2, and NaAl11O17 with acidic anodizing wastewater, and then precipitating the electrolyte components from the mixed filtrate from the previous two steps. The influences of stirring rate, liquid–solid ratio, temperature, and time on the extent of leaching of cryolite and recovery of carbon were studied. Additionally, the effects of pH value, F/Al ratio, temperature, and time on the recovery of valuable components in the mixed filtrate were evaluated. The results showed that most NaF in the SPL was dissolved by water leaching. The residual electrolyte in SPL was mainly cryolite and contained approximately 0.95 pct NaF. The purity of the carbon obtained reached 95.5 pct under optimal experimental conditions (leaching temperature: 80 °C; stirring rate: 300 rpm; liquid–solid ratio: 8 mL/g; leaching time: 180 minutes). The recovery of cryolite and the purity of the sodium sulfate crystal from the mixed filtrate were 98.4 and 92.0 pct, respectively, under suitable conditions (pH 9; 75 °C; 4 hour; F/Al ratio of 6:1).















Similar content being viewed by others
References
Y. Courbariaux, J. Chaouki, and C. Guy: Ind. Eng. Chem. Res., 2004, vol. 43, pp. 5828-5837.
V. Gomes, P. Z. Drumond, J. O. P. Neto, and A. R. Lira: Light Metals, TMS, Warrendale, 2005, pp. 1057-1063.
D. Miksa, M. Homsak, and N. Samec: Waste Manage. Res., 2003, vol. 21, pp. 467-473.
R.P. Pawlek: Light Metals, TMS, Warrendale, 2018, pp. 671-674.
Z. Shi, W. Li, X. Hu, B. Ren, B. Gao, and Z. Wang: Trans. Nonferrous Met. Soc. China, 2012, vol. 22, pp. 222-227.
D. F. Lisbona, C. Somerfield, and K. M. Steel: Hydrometallurgy, 2013, vols. 134-135, pp. 132-143.
J. A. Camargo: Chemosphere, 2003, vol. 50, pp. 251-264.
X. Zhao and L. Ma: IOP Conf. Ser., 2018, 108, 042023
S. S. Parhi: Master’s Thesisi, National Institute of Technology, Rourkela, Odisha, 2014, p. 12
TMS, P. von Krüger: Light Metals, TMS, 2011, pp. 275-280.
D. Yu, and K. Chattopadhyay: Int. J. Miner. Metall. Mater., 2018, vol. 25, pp.881-891.
D. Yu, V. Mambakkam, A. H. Rivera, D. Li and K. Chattopadhyay: Aluminium International Today, 2015, pp. 5
G. Holywell, R. Breault: JOM, 2013, vol. 65, pp. 1441-1451.
V. Y. Bazhin and R. K. Patrin: Refract. Ind. Ceram, 2011, vol. 52, pp. 63-65.
G. Hamel, R. Breault, G. Charest, S. Poirier, and B. Boutin: Light Metals, TMS, Warrendale, 2009, pp. 921-925.
W. Li and X. Chen: Light Metals, TMS, Warrendale, 2010, pp. 1064-1066.
C. A. Young, S. Nordwick, and M. Foote: The Fourth International Conference on Materials Engineering for Resources, Akita, Japan, 2001, pp. 13-25.
K. Mansfield, G. Swayn, J. Harpley, and P. R. Tayllor: EPD Congress: Fundamentals of Advanced Materials for Energy Conversion, Seattle, USA, 2002, p. 315-27.
J. F. Bush: Light Metals, TMS, Warrendale, 1986, pp. 1081-1099.
L. Pulvirenti, C. W. Mastropietro, A. Barkatt, and S. M. Finger: J. Hazard. Mater, 1996, vol. 46, pp. 13-21.
D. F. Lisbona, S. Christopher, and M. S. Karen: Ind. Eng. Chem. Res., 2012, vol. 51, pp. 8366-8377.
B. S. Scott, D. T. Brett, and W. S. Scott: J. Environ. Chem. Eng., 2015, vol. 3, pp. 2580-2587.
X. Z. Cao, Y. Y. Shi,S. Zhao, and X. X. Xue: J. Northeast. Univ. Nat. Sci., 2014, 35, 1746-1749.
D. F. Lisbona and M. S. Karen: Sep. Purif. Technol., 2008, vol. 61, pp. 182-192.
D. F. Lisbona, C. Somerfield, and K. M. Steel: Ind. Eng. Chem. Res., 2012, vol. 51, pp. 12712-12722.
S. L. Cheng. Master’s Thesis, Nanchang University, Nanchang, 2008, p. 29.
X. H. Liu, X. M. Zhang, S. L. Cheng, and J. J. Chen: Light Metals, 2011, vol. 40, pp. 15-18.
Acknowledgments
We thank the National Natural Science Foundation of China (Nos. 51574189, 51774224, 51574191) for financial support for this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Manuscript submitted August 10, 2018.
Rights and permissions
About this article
Cite this article
Li, X., Yin, W., Fang, Z. et al. Recovery of Carbon and Valuable Components from Spent Pot Lining by Leaching with Acidic Aluminum Anodizing Wastewaters. Metall Mater Trans B 50, 914–923 (2019). https://doi.org/10.1007/s11663-018-1485-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1485-3