Abstract
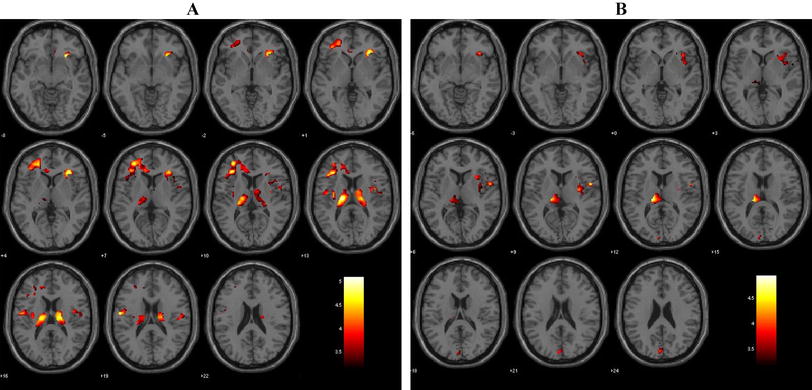
The lateral and third ventricles, as well as the corpus callosum (CC), are known to be affected in schizophrenia. Here we investigate whether abnormalities in the lateral ventricles (LVs), third ventricle, and corpus callosum are related to one another in first episode schizophrenia (FESZ), and whether such abnormalities show progression over time. Nineteen FESZ and 19 age- and handedness-matched controls were included in the study. MR images were acquired on a 3-Tesla MRI at baseline and ~1.2 years later. FreeSurfer v.5.3 was employed for segmentation. Two-way or univariate ANCOVAs were used for statistical analysis, where the covariate was intracranial volume. Group and gender were included as between-subjects factors. Percent volume changes between baseline and follow-up were used to determine volume changes at follow-up. Bilateral LV and third ventricle volumes were significantly increased, while central CC volume was significantly decreased in patients compared to controls at baseline and at follow-up. In FESZ, the bilateral LV volume was also inversely correlated with volume of the central CC. This inverse correlation was not present in controls. In FESZ, an inverse correlation was found between percent volume increase from baseline to follow-up for bilateral LVs and lesser improvement in the Global Assessment of Functioning score. Significant correlations were observed for abnormalities of central CC, LVs and third ventricle volumes in FESZ, suggesting a common neurodevelopmental origin in schizophrenia. Enlargement of ventricles was associated with less improvement in global functioning over time.




Similar content being viewed by others
References
Alexander-Bloch, A. F., Vertes, P. E., Stidd, R., Lalonde, F., Clasen, L., Rapoport, J., et al. (2013). The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cerebral Cortex, 23(1), 127–138. doi:10.1093/cercor/bhr388.
Andreasen, N. C. (1983). Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa.
Andreasen, N. C. (1984). Scale for the Assessment of Positive Symptoms. Iowa City: University of Iowa.
Arnone, D., McIntosh, A. M., Tan, G. M., & Ebmeier, K. P. (2008). Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophrenia Research, 101(1–3), 124–132. doi:10.1016/j.schres.2008.01.005.
Bergen, S. E., & Petryshen, T. L. (2012). Genome-wide association studies of schizophrenia: does bigger lead to better results? Current Opinion in Psychiatry, 25(2), 76–82. doi:10.1097/YCO.0b013e32835035dd.
Brent, B. K., Thermenos, H. W., Keshavan, M. S., & Seidman, L. J. (2013). Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia: a review of structural MRI findings. Child and Adolescent Psychiatric Clinics of North America, 22(4), 689–714. doi:10.1016/j.chc.2013.06.003.
Cahn, W., van Haren, N. E., Hulshoff Pol, H. E., Schnack, H. G., Caspers, E., Laponder, D. A., et al. (2006). Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. The British Journal of Psychiatry, 189, 381–382. doi:10.1192/bjp.bp.105.015701.
Del Re, E. C., Bergen, S. E., Mesholam-Gately, R. I., Niznikiewicz, M. A., Goldstein, J. M., Woo, T. U., et al. (2014). Analysis of schizophrenia-related genes and electrophysiological measures reveals ZNF804A association with amplitude of P300b elicited by novel sounds. Transcultural Psychiatry, 4, e346. doi:10.1038/tp.2013.117.
del Re, E. C., Gao, Y., Eckbo, R., Petryshen, T. L., Blokland, G. A. M., Seidman, L. J., Konishi. J., Goldstein, J. M., McCarley, R. W., Shenton, M. E., Bouix, S. (2015a). A new MRI masking technique based on multi-atlas brain segmentation in controls and schizophrenia: a rapid and Viable alternative to manual masking. Journal of Neuroimaging, doi:10.1111/jon.12313
del Re, E. C., Spencer, K. M., Oribe, N., Mesholam-Gately, R. I., Goldstein, J., Shenton, M. E., et al. (2015b). Clinical high risk and first episode schizophrenia: auditory event-related potentials. Psychiatry Research, 231(2), 126–133. doi:10.1016/j.pscychresns.2014.11.012.
DeLisi, L. E., Hoff, A. L., Kushner, M., Calev, A., & Stritzke, P. (1992). Left ventricular enlargement associated with diagnostic outcome of schizophreniform disorder. Biological Psychiatry, 32(2), 199–201.
Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191.
First, M. B., & Pincus, H. A. (2002). The DSM-IV text revision: rationale and potential impact on clinical practice. Psychiatric Services, 53(3), 288–292.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355.
Fitzsimmons, J., Kubicki, M., & Shenton, M. E. (2013). Review of functional and anatomical brain connectivity findings in schizophrenia. Current Opinion in Psychiatry, 26(2), 172–187.
Francis, A. N., Bhojraj, T. S., Prasad, K. M., Kulkarni, S., Montrose, D. M., Eack, S. M., et al. (2011). Abnormalities of the corpus callosum in non-psychotic high-risk offspring of schizophrenia patients. Psychiatry Research, 191(1), 9–15. doi:10.1016/j.pscychresns.2010.09.007.
Fusar-Poli, P., Smieskova, R., Kempton, M. J., Ho, B. C., Andreasen, N. C., & Borgwardt, S. (2013). Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neuroscience and Biobehavioral Reviews, 37(8), 1680–1691. doi:10.1016/j.neubiorev.2013.06.001.
Green, M. J., Cairns, M. J., Wu, J., Dragovic, M., Jablensky, A., Tooney, P. A., et al. (2013). Genome-wide supported variant MIR137 and severe negative symptoms predict membership of an impaired cognitive subtype of schizophrenia. Molecular Psychiatry, 18(7), 774–780. doi:10.1038/mp.2012.84.
Hien, D., Matzner, F. J., First, M. B., Sptizer, R. L., Gibbon, M., Williams, J. B. W. (1994). Structured clinical interview for DSM-IV-child edition (version 1.0). New York: Columbia University.
Hofer, S., & Frahm, J. (2006). Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage, 32(3), 989–994. doi:10.1016/j.neuroimage.2006.05.044.
Hollingshead, A. B. (1975). Two-factor index of social position. New Haven, CT: Yale University Press
Johnson, S. L., Greenstein, D., Clasen, L., Miller, R., Lalonde, F., Rapoport, J., et al. (2013). Absence of anatomic corpus callosal abnormalities in childhood-onset schizophrenia patients and healthy siblings. Psychiatry Research, 211(1), 11–16. doi:10.1016/j.pscychresns.2012.09.013.
Johnstone, E. C., Crow, T. J., Frith, C. D., Husband, J., & Kreel, L. (1976). Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet, 2(7992), 924–926.
Jones, S. H., Thornicroft, G., Coffey, M., & Dunn, G. (1995). A brief mental health outcome scale-reliability and validity of the global assessment of functioning (GAF). The British Journal of Psychiatry, 166(5), 654–659.
Kempton, M. J., Stahl, D., Williams, S. C., & DeLisi, L. E. (2010). Progressive lateral ventricular enlargement in schizophrenia: a meta-analysis of longitudinal MRI studies. Schizophrenia Research, 120(1–3), 54–62. doi:10.1016/j.schres.2010.03.036.
Kubicki, M., & Shenton, M. E. (2015). Editorial to special issue on "white matter pathology". Schizophrenia Research, 161(1), 1–3. doi:10.1016/j.schres.2014.12.015.
Lazo, D. L. (2004). Fundamentals of sectional anatomy: an imaging approach, 1st Edition. Stamford: Cengage Learning.
Lett, T. A., Chakravarty, M. M., Felsky, D., Brandl, E. J., Tiwari, A. K., Goncalves, V. F., et al. (2013). The genome-wide supported microRNA-137 variant predicts phenotypic heterogeneity within schizophrenia. Molecular Psychiatry, 18(4), 443–450. doi:10.1038/mp.2013.17.
Mitelman, S. A., & Buchsbaum, M. S. (2007). Very poor outcome schizophrenia: clinical and neuroimaging aspects. International Review of Psychiatry, 19(4), 345–357. doi:10.1080/09540260701486563.
Mitelman, S. A., Nikiforova, Y. K., Canfield, E. L., Hazlett, E. A., Brickman, A. M., Shihabuddin, L., et al. (2009). A longitudinal study of the corpus callosum in chronic schizophrenia. Schizophrenia Research, 114(1–3), 144–153. doi:10.1016/j.schres.2009.07.021.
Nakamura, M., Salisbury, D. F., Hirayasu, Y., Bouix, S., Pohl, K. M., Yoshida, T., et al. (2007). Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biological Psychiatry, 62(7), 773–783. doi:10.1016/j.biopsych.2007.03.030.
Narr, K. L., Thompson, P. M., Sharma, T., Moussai, J., Cannestra, A. F., & Toga, A. W. (2000). Mapping morphology of the corpus callosum in schizophrenia. Cerebral Cortex, 10(1), 40–49.
Narr, K. L., van Erp, T. G., Cannon, T. D., Woods, R. P., Thompson, P. M., Jang, S., et al. (2002). A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiology of Disease, 11(1), 83–95.
Nasrallah, H. A., Jacoby, C. G., Chapman, S., & McCalley-Whitters, M. (1985). Third ventricular enlargement on CT scans in schizophrenia: association with cerebellar atrophy. Biological Psychiatry, 20(4), 443–450.
Pfefferbaum, A., Sullivan, E. V., & Carmelli, D. (2004). Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiology of Aging, 25(2), 175–183.
Pfefferbaum, A., Sullivan, E. V., Swan, G. E., & Carmelli, D. (2000). Brain structure in men remains highly heritable in the seventh and eighth decades of life. Neurobiology of Aging, 21(1), 63–74.
Rotarska-Jagiela, A., Schonmeyer, R., Oertel, V., Haenschel, C., Vogeley, K., & Linden, D. E. (2008). The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. NeuroImage, 39(4), 1522–1532. doi:10.1016/j.neuroimage.2007.10.063.
Salat, D. H., Tuch, D. S., Greve, D. N., van der Kouwe, A. J., Hevelone, N. D., Zaleta, A. K., et al. (2005). Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging, 26(8), 1215–1227. doi:10.1016/j.neurobiolaging.2004.09.017.
Schizophrenia Psychiatric Genome-Wide Association Study, C (2011). Genome-wide association study identifies five new schizophrenia loci. Nature Genetics, 43(10), 969–976. doi:10.1038/ng.940.
Schizophrenia Working Group of the Psychiatric Genomics, C (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427. doi:10.1038/nature13595.
Serpa, M. H., Schaufelberger, M. S., Rosa, P. G., Duran, F. L., Santos, L. C., Muray, R. M., et al. (2012). Corpus callosum volumes in recent-onset schizophrenia are correlated to positive symptom severity after 1 year of follow-up. Schizophrenia Research, 137(1–3), 258–259. doi:10.1016/j.schres.2012.02.027.
Shenton, M. E., Dickey, C. C., Frumin, M., & McCarley, R. W. (2001). A review of MRI findings in schizophrenia. Schizophrenia Research, 49(1–2), 1–52.
Silber, J., Lim, D. A., Petritsch, C., Persson, A. I., Maunakea, A. K., Yu, M., et al. (2008). miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Medicine, 6(14). doi:10.1186/1741-7015-6-14.
Stoll, A. L. (2009). The psychopharmacology reference card, 1989–2009. Belmont, MA: McLean Hospital.
van Haren, N. E., Hulshoff Pol, H. E., Schnack, H. G., Cahn, W., Brans, R., Carati, I., et al. (2008). Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biological Psychiatry, 63(1), 106–113.
Wechsler, D. (1999). Wechsler abbreviated scale of intelligence. New York: Psychological Corporation, Harcourt Brace.
Whitford, T. J., Ford, J. M., Mathalon, D. H., Kubicki, M., & Shenton, M. E. (2012). Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schizophrenia Bulletin, 38(3), 486–494. doi:10.1093/schbul/sbq105.
Whitford, T. J., Kubicki, M., Schneiderman, J. S., O’Donnell, L. J., King, R., Alvarado, J. L., et al. (2010). Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biological Psychiatry, 68(1), 70–77. doi:10.1016/j.biopsych.2010.03.025.
Wilkinson, G. S, Robertson, G. J. (2006). Wide range achievement test, Fourth edition. Lutz, FL: Psychological Assessment Resources.
Witelson, S. F. (1989). Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain, 112 (Pt 3), 799–835.
Woodruff, P. W., McManus, I. C., & David, A. S. (1995). Meta-analysis of corpus callosum size in schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry, 58(4), 457–461.
Woods, S. W. (2003). Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry, 64(6), 663–667.
Wright, C., Calhoun, V. D., Ehrlich S., Wang L., Turner J. A., and Bizzozero N. I. (2015). Meta gene set enrichment analyses link miR-137-regulated pathways with schizophrenia risk. Frontiers in Genetics, 6, 147.
Acknowledgments
We thank all subjects who participated in the study. We also thank the clinical, research assistant, and data management staff from the Boston CIDAR study, including.
Ann Cousins, PhD, APRN, Michelle Friedman-Yakoobian, PhD, Anthony J. Giuliano, PhD, Matcheri Keshavan, MD, Janine Rodenhiser-Hill, PhD, Kristen A. Woodberry, MSW, PhD, Andréa Gnong-Granato,MSW, Lauren Gibson, EdM, Sarah Hornbach, BA, Julia Schutt,BA, Kristy Klein, PhD, Maria Hiraldo, PhD, Grace Francis, PhD, Corin Pilo, LMHC, Rachael Serur, BS, Reka Szent-Imry, BA, Shannon Sorenson, BA, Grace Min, EdM, Alison Thomas, BA, Chelsea Wakeham, BA, Caitlin Bryant, BS, and Molly Franz, BA. Finally, we are grateful for the hard work of many research volunteers, including Zach Feder, Elizabeth Piazza, Julia Reading, Devin Donohoe, Sylvia Khromina, Alexandra Oldershaw, and Olivia Schanz.
Contributors
E.C. del Re designed this study, analyzed the data, and wrote the manuscript; J. Konishi helped with data analysis; S. Bouix helped with MRI tools and edited the manuscript; G. Blokland, O. Pasternak, J. Goldstein, M. Kubicki, Y. Hirayasu and Margaret Niznikiewicz edited the manuscript; R. Mesholam-Gately and L. Seidman recruited the subjects, collected clinical data, edited the manuscript and contributed funding (LJS); T. Petryshen edited the manuscript and contributed funding for this project; M. Shenton and R. McCarley contributed funding for this research project and contributed to manuscript writing and data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Role of funding source
This study was conducted in part by the P50 MH080272 Boston Center for Intervention Development and Applied Research (Boston CIDAR; RWM PI, MES, LJS, RI-M-G, JG, TLP), entitled: “Longitudinal Assessment and Monitoring of Clinical Status and Brain Function in Adolescents and Adults.” This work was also supported in part by R01 MH40799 (RWM) and R01MH092380 (TLP) from National Institutes of Health, by the Commonwealth Research Center (SCDMH82101008006, LJS), and by a Clinical Translational Science Award UL1RR025758 to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources (LJS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interests
All authors declare that they do not have conflicts of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
del Re, E.C., Konishi, J., Bouix, S. et al. Enlarged lateral ventricles inversely correlate with reduced corpus callosum central volume in first episode schizophrenia: association with functional measures. Brain Imaging and Behavior 10, 1264–1273 (2016). https://doi.org/10.1007/s11682-015-9493-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-015-9493-2