Abstract
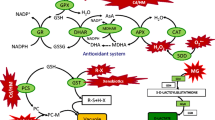
The protective effects of spermine (SPM) and putrescine (PUT) against paraquat (PQ), a herbicide in agriculture and oxidative stress inducer, were investigated in the leaves of maize. Maize leaves were pretreated to SPM and PUT at concentrations of 0.2 and 1 mM and treated with PQ afterwards. Pretreatment with 1 mM of SPM and PUT significantly prevented the losses in chlorophyll and carotenoid levels induced by PQ. Ascorbic acid content in the leaves pretreated with both polyamines was found to be higher than those of the leaves pretreated with water. Also, pretreatment with SPM and PUT was determined to have some effects on the activities of superoxide dismutase (SOD) and peroxidase (POD). 1 mM of SPM increased SOD activity, but PUT has no significant effect on SOD activity. On the other hand, POD activity was recorded to increase slightly in response to both concentrations of SPM and 1 mM of PUT. The results showed that such polyamine pretreated plants may become more tolerant to oxidative stress due to increases in the antioxidative enzymes and antioxidants.
Similar content being viewed by others
Abbreviations
- EDTA:
-
Ethylenediamine tetra acetic acid
- NBT:
-
Nitroblue tetrazolium
- POD:
-
Peroxidase
- PQ:
-
Paraquat
- PUT:
-
Putrescine
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- SPM:
-
Spermine
References
Arnon D.I. 1949. Copper enzymes in chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol. 24: 1–15.
Asada K., Kiso K.. 1973. Initiation of aerobic oxidation of sulfite by illuminated spinach chloroplast. Eur. J. Biochem. 33: 253–257.
Bais H.P., Ravishankar G.S. 2002. Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell, Tissue and Organ Culture 69: 1–34.
Beauchamp C., Fridovich I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 44: 276–287.
Besford R.T., Richardson C.M., Campos J.L., Tiburcio A.F. 1993. Effect of polyamines on stabilization of molecular complexes of thylakoid membranes of osmotically stressed oat leaves. Planta 189: 201–206.
Bors W., Langebartels C., Michel C., Sandermann J.H. 1989. Polyamines as radical scavengers and protectants against ozone damage. Phytochem. 28: 1589–1595.
Bratton D.L. 1994. Polyamine inhibition of transbilayer movement of plasma membrane phospholipids in the erythrocyte ghost. J. Biol. Chem. 269: 22517–22523.
Cakmak I., Marschner H. 1992. Magnesium deficiency enhances resistance to paraquat toxicity in bean leaves. Plant, Cell and Environment 15: 955–960.
Canal M.J., Tames R.S., Fernandez B. 1988. Peroxidase and polyphenol oxidase activities in Cyperus esculentus leaves following glyphosate applications. Physiol. Plant. 74: 125–130.
Carlioz A., Toutai D. 1986. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? The EMBO Journal 5: 623–630.
Demming-Adams B. 1990. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochem. Biophys. Acta 1020: 1–24.
Dhindsa R.S., Matowe W. 1981. Drought tolerance in two mosses: Correlated with enzymatic defense against lipid peroxidation. J. Exp. Bot. 32: 79–91.
Dodge A. 1994. Herbicide action and effects on detoxification processes. In: Foyer C.H. and Mullineaux P.M. (eds), Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, FL, pp. 219–236.
Drolet G., Dumbroff E.B., Legge R.L., Thompson J.E. 1986. Radical scavenging properties of polyamines. Phytochem. 25: 367–371.
Furusawa I., Tanaka K., Thanutong P., Mizuguchi M., Yazaki M., Asada K. 1984. Paraquat resistant tobacco calluses with enhanced superoxide dismutase activity. Plant and Cell Physiol. 25: 1247–1254.
Gupta A.S., Webb R.P., Holaday S.A., Allen R.D. 1993. Overexpression of superoxide dismutase protects plants from oxidative stress. Plant Physiol. 103: 1067–1073.
Halliwell B. 1982. The toxic effects of oxygen on plant tissues. In: Oberley L.W. (ed), Superoxide dismutase. CRC Press, Boca Raton, Florida, pp. 89–124.
Jaspars E.M.J. 1965. Pigmentation of tobacco crowngall tissues cultured in vitro in dependence of the composition of the medium. Physiol. Plant. 18: 933–940.
Kenylon W.H., Duke S.D. 1985. Effects of acifluorfen on endogenous antioxidants and protective enzymes in cucumber. Plant Phsiol. 79: 216–220.
Kirtikara K., Talbot D. 1996. Alteration in protein accumulation, gene expression and ascorbate- glutathione pathway in tomato (Lycopersicon esculentum) under paraquat and ozone stress. J. Plant Physiol. 148: 752–760.
Knox J.P., Dodge A.D. 1985. Singlet oxygen and plants. Phytochem. 24: 889–896.
Langebartels C., Kerner K., Leonardi S., Schraudner M., Trost M., Heller W., Sandermann H. 1991. Biochemical plant responses to ozone. Differential induction of polyamine and ethylene biosynthesis in tobacco, Plant Physiol. 95: 882–889.
Liebler D.C., Kling D.S., Reed D.J. 1986. Antioxidant protection of phospholipid bilayers by á- tocopherol. Control of a-tocopherol status by ascorbic acid and glutathione, J.Biol. Chem. 2061:12114–12119.
Martin-Tanguy J. 2001. Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul. 34: 135–148.
Mehlhorn H. 1990. Ethylene- promoted ascorbate peroxidase activity protects plants against hydrogen peroxide, ozone and paraquat. Plant, Cell and Environ. 13: 971–976.
Noctor G., Arisi A.M., Jouanin L., Kunert K.J., Rennenberg H., Foyer C. 1998. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 49: 623–647.
Noguchi T., Hayashi H., Tasumi H. 1990. Factors controlling the efficiency of energy transfer from carotenoids to bacteriochlorophyll in purple photosynthetic bacteria. Biochem. Biophys. Acta. 1017: 280–290.
Ormrod D.P., Beckerson D.W. 1986. Polyamines as antiozonants for tomato. Hort. Scien. 21: 1070–1071.
Padh H. 1990. Cellular functions of ascorbic acid. Biochem. and Cell Biol. 68: 1166–1173.
Panagiotidis C.A., Artandi S., Calame K., Silverstein S.J. 1995. Polyamines alter sequence-specific DNA protein interactions. Nucleic Acids Res. 23: 1800–1809.
Pastori G.M., Trippi V.S. 1992. Oxidative stress induces high rate of glutathione reductase synthesis in a drought-resistant maize strain. Plant Cell Physiol. 33: 957–961.
Rodriguez R., Sanchez T.R. 1982. Peroxidase and IAA oxidase in germinating seeds of Cicer arietinum L. Rev. Esp. Fisiol. 38: 183–188.
Sakaki T., Kondo N., Sugahara K. 1983. Breakdown of photosynthetic pigments and lipids in spinach leaves with ozone fumigation: role of active oxygens. Phsiol. Plant. 50: 28–34.
Sreenivasulu N., Ramanjulu S., Ramachandra-Kini K., Prakash H.S., Shekar-Shetty H., Savithri H.S. 1999. Total peroxidase activity and peroxidase isoforms as modified by salt stress in two cultivars of foxtail millet with differential salt tolerance. Plant Science 141:1–9.
Shaaltiel Y., Glazer A., Bocion P.F., Gressel J. 1988. Cross tolerance to herbicidal and environmental oxidants of plant biotypes tolerant to paraquat, sulfur dioxide and ozone. Pest. Biochem. and Physiol. 31: 13–23.
Shieh H.H., Sweet T.R. 1979. Spectrophotometric determination of ascorbic acid. Anal. Biochem. 96: 1–5.
Tadolini B. 1988. Polyamine inhibition of lipoperoxidation: The influence of polyamines on iron oxidation in the presence of compounds mimicking phospholipid polar heads. Biochem J. 249: 33–36.
Tanaka K., Furusawa I., Kondo N., Tanaka K. 1988. SO2 tolerance of tobacco plants regenerated from paraquat-tolerant callus. Plant Cell Physiol. 29: 743–746.
Thompson J.E., Ledge R.L., Barber R.F. 1987. The role of free radicals in senescence and wounding. New Phytol. 105: 317–344.
Tiburcio A.F., Altabella T., Borrel T., Masgrau C. 1997. Polyamines metabolism and its regulation. Physiol. Plant. 100: 664–674.
Velikova V., Yordanov I., Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Science 151: 59–66.
Ye B., Müller H.H., Zhang J., Gressel J. 1997. Constitutively elevated levels of putrescine and putrescinegenerating enzymes correlated with oxidant stress resistance in Conyza bonariensis and wheat. Plant Physiol. 115: 1443–1451
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Durmu, N., Kadioğlu, A. Spermine and putrescine enhance oxidative stress tolerance in maize leaves. Acta Physiol Plant 27, 515–522 (2005). https://doi.org/10.1007/s11738-005-0057-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-005-0057-8