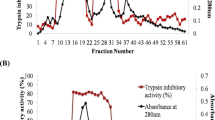
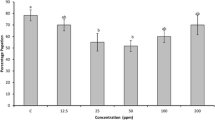
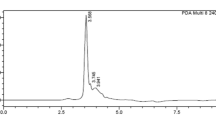
Abstract
Protease inhibitors present in seeds of legumes possess strong inhibitory activity against trypsin and confer resistance against pests. In the present investigation, trypsin inhibitor activity was found in the seed flour extracts of all the eight selected varieties of mungbean under study which was further confirmed by dot blot analysis. All the varieties showed inhibitory activity in vitro against the gut protease of Helicoverpa armigera (HGP). Trypsin inhibitor was purified from mungbean seeds to near homogeneity with 58.1-fold and 22.8% recovery using heat denaturation, NH4(SO4)2 fractionation, ion-exchange chromatography on DEAE-Sephadex A-25 and gel filtration through Sephadex G-75. The molecular mass of the inhibitor was 47 kDa as determined by gel filtration and SDS-PAGE. The inhibitor retained 90% or more activity between pH 4 and 10, however, it was nearly inactive at extreme pH values. The inhibitor was stable up to 80°C but thereafter, the activity decreased gradually retaining nearly 30% of activity when heated at 100°C for 20 min. The inhibitor activity was undetectable at 121°C. Insect bioassay experiment using purified mungbean trypsin inhibitor showed a marked decline in survival (%) of larvae with increase in inhibitor concentration. The larval growth was also extended by the trypsin inhibitor. This study signifies the insecticidal potential of mungbean trypsin inhibitor which might be exploited for raising transgenic plants.










Similar content being viewed by others
References
Annapurna SS, Ramadoss CS, Prasad DS (1991) Characterization of a trypsin/chymotrypsin inhibitor from jack fruit (Artocarpus integrifolia) seeds. J Sci Food Agric 54:605–618
Broadway RM (1995) Are insect resistant to plant proteinase inhibitors? J Insect Physiol 41:107–116
Chrispeels MJ, Baumgartner B (1978) Trypsin inhibitor in mungbean cotyledons. Plant Physiol 61:617–623
Davis BJ (1964) Disc electrophoresis. II. Method and application to human serum proteins. Ann N Y Acad Sci 121:404–427
deLeo F, Gallerani R (2002) The mustard trypsin inhibitor 2 affects the fertility of Spodoptera littorallis larvae fed on transgenic plants. Insect Biochem Mol Biol 32:489–496
Dellaporta SL, Wood J, Hecks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19
Felicioli R, Garzelli B, Vaccari L, Melfi D, Balestreri E (1997) Activity staining of protein inhibitors of proteases on gelatin-containing polyacrylamide gel electrophoresis. Anal Biochem 244:176–179
Ferrasson E, Quillien L, Gueguen J (1997) Proteinase inhibitors from pea seeds: purification and characterization. J Agric Food Chem 45:127–131
Gatehouse AMR, Gatehouse JA (1998) Identifying proteins with insecticidal activity: use of encoding genes to produce insect-resistant transgenic crops. Pest Sci 52:165–175
Gatehouse AMR, Gatehouse JA, Boulter D (1980) Isolation and characterization of trypsin inhibitor from cowpea (Vigna unguiculata). Phytochemistry 19:751–756
Gatehouse AMR, Norton E, Davis GM, Babbe SM, Newell CA, Gatehouse JA (1999) Digestive proteolytic activity in larvae of tomato moth, Lacanobia oleracea; effects of plant proteinase inhibitor in vitro and in vivo. J Insect Physiol 45:545–558
Ghorpade VM, Kadam SS, Salunkhe DK (1986) Thermal stability and changes in trypsin inhibitor during germination and cooking of horse gram. J Food Sci Technol 23:164–165
Giri AP, Harsulkar AM, Ku MSB, Gupta VS, Deshpande VV, Ranjekar PK, Franceschi VR (2003) Identification of potent inhibitors of Helicoverpa armigera gut proteinases from winged bean seeds. Phytochemistry 63:523–532
Godbole SA, Krishna TG, Bhatia CR (1994) Purification and characterization of protease inhibitors from pigeon pea (Cajanus cajan) seeds. J Sci Food Agric 64:87–93
Gomes APG, Dias SC, Bloch C Jr, Melo FR, Furtado JR Jr, Monnerat RG, Grossi-de-Sá MF, Franco OL (2005) Toxicity to cotton boll weevil Anthonomus grandis of a trypsin inhibitor from chickpea seeds. Comp Biochem Physiol B 140:313–319
Gupta P, Dhawan K, Malhotra SP, Singh R (2000) Purification and characterization of trypsin inhibitor from seeds of faba bean (Vicia faba L). Acta Physiol Plant 22:433–438
Hajela N, Sharma AH, Sharma DN, Rao S, Hajela K (1999) Studies on a doubleheaded protease inhibitor from Phaseolus mungo. J Plant Biochem Biotechnol 8:57–60
Harsulkar AM, Giri AP, Patankar AG, Gupta VS, Sainani MN, Ranjekar PK, Despande VV (1999) Successive use of non-host plant proteinase inhibitors required for effective inhibition of Helicoverpa armigera gut proteinases and larval growth. Plant Physiol 121:497–506
Johnston KA, Gatehouse JA, Anstee JH (1991) In vitro and In vivo studies of the effects of plant proteinases inhibitors on Helicoverpa armigera larvae. J Exp Bot 42:238
Johnston KA, Gatehouse JA, Anstee JH (1993) Effect of soybean protease inhibitors on the growth and development of larval Helicoverpa armigera. Insect Physiol 39:657–664
Jouanin L, Bonade-Bottino M, Girard C, Morrot G, Giband M (1998) Transgenic plants for insect resistance. Plant Sci 131:1–11
Kakade ΜL, Simons Ν, Liener ΙΕ (1969) The evaluation of natural vs synthetic substrates for measuring the antitrypsin activities of soybean samples. Cereal Chem 46:518–526
Kunitz M (1945) Crystallization of a trypsin inhibitor from soybean. Science 101:668
Laemmli EK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laskowski M Jr, Qasim MA (2000) What can the structures of enzyme–inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochem Biophys Acta 1477:324–337
Lawn RJ, Ahn CS (1985) Mung bean (Vigna radiata (L) Wilczek/Vigna mungo (L) Hepper). In: Summerfield RJ, Roberts EH (eds) Grain legume crops. William Collins Sons & Co Ltd, London, pp 584–623
Lawrence JC, Nielsen SS (2001) Partial characterization of a cysteine proteinase inhibitor from Lima bean (Phaseolus lunatus). J Agric Food Chem 49:1020–1025
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:1265–1275
Maggo S, Malhotra SP, Dhawan K, Singh R (1999) Purification and characterization of protease inhibitor from rice bean (Vigna umbellate). J Plant Biochem Biotechnol 8:61–64
Murdock LL, Shade RE (2002) Lectins and protease inhibitors as plant defenses against insects. J Agric Food Chem 50:6605–6611
Ryan CA (1991) Proteinase inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Plant Physiol 28:425–449
Saini HS (1989) Thermal stability of protease inhibitors in some cereals and legumes. Food Chem 32:59–67
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, USA
Singh A, Dudey A, Tyagi TK, Gaur AK, Mishra DP (2005) Screening of different Indian wheat varieties for alpha amylase inhibitor proteins/genes to develop insect-resistant transgenic plants. J Plant Biol 32:149–153
Solomon M, Belenghi B, Delledone M, Menachem E, Levine A (1999) The involvement of cysteine proteinase and proteinase inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11:431–443
Sudheendra K, Mulimani VH (2002) Effect of feeding legume proteinase inhibitors on Helicoverpa armigera gut proteinase activity. ICPN 9:51–53
Telang M, Srinivasan A, Patankar A, Harsulkar A, Joshi V, Damle A, Deshapande V, Sainani M, Ranjekar P, Gupta G, Birah A, Rani S, Kachole M, Giri A, Gupta V (2003) Bitter gourd proteinase inhibitors: potential growth inhibitors of Helicoverpa armigera and Spodoptera litura. Phytochemistry 63:643–652
Veera Reddy GG, Bhattacharya AK (1990) Development of semi-synthetic diets for the rearing of Heliothis armigera (Hub). J Insect Sci 3:23–33
Whitley EJ, Bowman DE (1975) Isolation and properties of navy beans proteinase inhibitor component I. Arch Biochem Biophys 169:42–50
Xavier-Filho J (1992) The biological roles of serine and cysteine proteinase inhibitors in plants. Braz J Plant Physiol 4:1–6
Acknowledgment
The authors want to thank National Agricultural Technology Project (Mission Mode), ICAR, New Delhi for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Klobus.
Rights and permissions
About this article
Cite this article
Kansal, R., Gupta, R.N., Koundal, K.R. et al. Purification, characterization and evaluation of insecticidal potential of trypsin inhibitor from mungbean (Vigna radiata L. Wilczek) seeds. Acta Physiol Plant 30, 761–768 (2008). https://doi.org/10.1007/s11738-008-0178-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-008-0178-y