Abstract
Since the publication of the RECOVERY trial, the use of glucocorticoid drugs (GC) has spread for the treatment of severe COVID-19 worldwide. However, the benefit of dexamethasone was largest in patients who received mechanical ventilation or supplemental oxygen therapy, while no benefit was found among patients without hypoxemia. In addition, a positive outcome was found in patients who received dexamethasone after several days of symptoms, while possible harm could exist if administered early. The right time interval for GC administration is still a matter of debate. Previous studies showed that an early GC use during the first phase of the disease, when viral replication peaks, may negatively affect the innate immune response through several mechanisms, such as the inhibition of pro-inflammatory and antiviral cytokine production and signaling pathway, including type I interferon, that is fundamental to counteract the virus and that was found to be impaired in several patients with life-threatening COVID-19. The GC misuse can lead to a more severe disease even in patients who do not have the established risk factors, such as obesity and cardiovascular diseases. In our focused review, we describe the role of immune response in viral infections, especially SARS-CoV-2, and discuss the potential harms of GC misuse in COVID-19.
Similar content being viewed by others
Introduction
Italy is one of the countries with the highest mortality rate for coronavirus disease 2019 (COVID-19), especially in subject aged 80 + . Since April 2021, after the beginning of the extensive vaccination campaign, especially to the older population, we have seen an almost steady decrease in the number of COVID-19 patients hospitalized or deceased compared to the previous period [1]. However, also due to the spread of SARS-CoV-2 variants, the battle against the virus is not yet won [2, 3].
In October 2020, the Italian Medicines Agency (Agenzia Italiana del Farmaco—AIFA) started to support the use of glucocorticoid drugs (GC), especially dexamethasone, in COVID-19 patients who required supplemental oxygen therapy and ventilation, mainly due to the positive findings of the RECOVERY trial [4]. Since then, the use of glucocorticoids (GC) has spread for the treatment of severe COVID-19 during the so-called “third wave of COVID-19” in Italy. Although there are recommendations on how to use GC in COVID-19 patients [5], several aspects of this therapy (i.e., dosages, time interval, patient selection) are still a matter of debate, needing more clinical trials to be clarified. In this context, GC misuse could be harmful for the patients, especially in the early phase of disease. In the following narrative review, we will discuss the possible implications of GC misuse in viral infections, especially COVID-19, from an immunological and a clinical point of view.
Methods
We searched Medline (PubMed) and Scopus to identify the published studies. The main search was run on March 2021 and updated until September 2021. We took into account observational studies (prospective or retrospective cohort, case–control or cross-sectional studies), case series and case reports, editorials, comments, clinical trials, review articles and meta-analyses published in the last 20 years. The search was limited to English language studies published in peer-reviewed journals. The keywords regarding the topics addressed (es. COVID-19, viral infection, glucocorticoids, immune system, cytokines, IL-6 antagonists) were typed in various combinations using Boolean operators (see the detailed keywords in supplemental material). Hand searches of reference lists of articles and relevant literature reviews were also used. For the preparation of this review, authors reviewed all the relevant articles found to ensure that they could address our review questions, and decided the studies to include by consensus, quality journals, articles with the most relevant data on the topic of interest and on the basis of our purpose, to make a comprehensive narrative synthesis of the available published information.
The role of innate and adaptive immune response against SARS-CoV-2 and other viral infections
The innate immune system provides the first line of defense against viruses [6]. Upon infecting cells in the respiratory tract, the single-strand RNA (ssRNA) structure of SARS-CoV-2 is recognized by Toll-like receptor 7 (TLR7) within endosomes, which leads to the induction of pro-inflammatory cytokines and interferons (IFN). In a large part of healthy individuals, this immune response is sufficient to clear the virus and prevent the development of severe disease. TLR7 was also a pattern recognition receptor for ssRNA of both MERS-CoV and SARS-CoV. It likely plays a key role also in SARS-CoV-2 infection, given that this coronavirus contains even more ssRNA motifs that could interact with TLR7 [6]. Rare putative loss-of-function variants of X-chromosomal TLR7 were detected in young men (age < 35 years) without preexisting medical conditions affected by severe COVID-19 [7]. Loss-of-function variants in TLR7 were associated with impaired type I and II IFN responses to coronavirus, that can lead to both impaired viral clearance and increased direct cytopathic viral effects due to the higher viral load [7]. Therefore, TLR7 function, together with type I and II IFN response, is likely to be of paramount importance to avoid severe COVID-19, which is largely related to viral load and persistence, resulting in diffuse damage of lung cells and plasma protein leakage into alveolar spaces [8]. Noteworthy, patients with severe COVID-19 exhibit impaired type I and type II IFN responses and lower viral clearance in clinical studies [9, 10]. Inborn errors of type I IFN immunity with loss-of-function, involving human loci known to govern TLR3 and interferon regulatory factor 7 (IRF7), were found in life-threatening COVID-19 patients [11]. Again, autoantibodies against interferons were found in critical patients, while these ones were not found in individuals with asymptomatic or mild SARS-CoV-2 infection [12]. Clinical trials on the administration of different interferon types have been conducted to evaluate the response to symptoms and outcomes in early phases of COVID-19, albeit with inconclusive results [13]. As regards adaptive immunity, SARS-CoV-2-specific antibodies, CD4 + T-cells, and CD8 + T-cells have protective role in controlling SARS-CoV-2 infection [14]. T-cell responses have been associated with control of primary infection. Among T-cells, CD4 + cells play a key role [14,15,16]. Indeed, if quickly inducted, since the first days post-symptom onset, SARS-CoV-2-specific CD4 + T-cells accelerate viral clearance and their level have the strongest association with attenuated COVID-19 disease severity, compared to antibodies and CD8 + T-cells [16, 17]. Virus-specific CD4 + T-cells can differentiate into a range of helper and effector cell types: Type-1 helper T-cells, which have direct antiviral activities producing IFN-γ and related cytokines, and T follicular helper cells, which provide B-cell maturation and development of most neutralizing antibodies, as well as memory B-cells [18]. In SARS-CoV-2 infection, also the high levels of virus-specific CD8 + T-cells have been associated with better COVID-19 outcomes, producing high levels of molecules with potent cytotoxic effector functions, such as IFN-γ, granzyme B, perforin, and CD107a [15, 16, 19]. CD8 cells are the most found among infiltrating cells in pathological examinations [20]. Even if T-cell have a protective role, their dysfunctional response accounted, in part, for the severe immune injury in COVID-19 with an exacerbated inflammatory response [21]. Neutralizing antibodies develop rapidly in most SARS-CoV-2-infected people. The vast majority seroconvert within 5–15 day post-symptoms onset [22] and spike protein is the main target of SARS-CoV-2 neutralizing antibodies. The presence of IgG to spike antigens correlates with time to a negative swab result, providing the best correlate of neutralization within the first weeks from symptoms onset [23]. However, around 1–2 weeks after symptoms onset, severely ill patients show a peak in inflammation, edema, and thrombosis. This is suggested not to be a direct effect of viral infection, but instead to be strictly related to seroconversion and to overactivation of adaptive immune response [24]. Indeed, it has been shown how anti-spike IgG from patients with severe COVID-19 has an increased inflammatory potential due to a different glycosylation, particularly low fucosylation of the antibody Fc tail [25]. Right after seroconversion, anti-spike IgG titers in these patients are higher and Fc glycosylation is most aberrant [24, 26]. “Pathogenic” anti-spike IgG amplify proinflammatory responses by human alveolar macrophages, through Fcγ Receptor (FcγR) IIa and FcγRIII activation, which induces cytokines production [27]. All this evidence emphasizes the key role played by innate and adaptive immunity, and its dysregulation, in controlling SARS-CoV-2 viral load and subsequent COVID-19 severity. Moreover, it could at least in part explain the wide inter-individual variability of SARS-CoV-2 clinical picture, ranging from silent infection to deadly disease, adding to the now well-known risk factors for worse outcomes, such as advanced age, obesity and cardiovascular diseases.
Possible harm related to the use of glucocorticoid drugs in viral infections
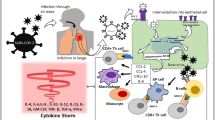
GC may affect the immune response to viral infections through several mechanisms. Indeed, they downregulate IFN-γ production, suppress antigen-stimulated inflammation mediated by macrophages and dendritic cells, impair cytotoxic immune responses of Type-1 helper T-cells, CD8 + T-cells, and natural killer (NK) cells [28]. GC inhibit pro-inflammatory (IL-6 and IL-8) and antiviral (IFN-1) cytokine production and signaling pathway, decreasing the expression of the IFN-stimulated genes [29, 30] (Fig. 1). These immune inhibitory effects, also depending on dosages, potency, and duration of treatment, may increase the susceptibility and severity of several infections. Therefore, if GC are given early in the course of a viral infection (e.g. within the first week of symptom onset when the innate response is mounting), they are likely to interfere and reduce both the efficacy of IFN production and IFN-mediated reduction of viral spread, enhancing viral replication [28, 31]. In addition, for these reasons, the clinical efficacy and safety of GC use in different viral pneumonia remains largely uncertain because of a lack of randomized trials and inconclusive observational studies. Although some reports found apparent beneficial effects of GC [32], others showed harmful effects, such as a reduced clearance of viral RNA in both severe acute respiratory syndrome (SARS) [33] and Middle East respiratory syndrome (MERS) outbreaks [34, 35]. Indeed, both Lee N. et al. and Arabi Y.M. et al. showed how the “early” initiation of GC therapy (within the first week of illness) resulted also in increased length of hospital stay and increased need for invasive ventilation in SARS and MERS, respectively [33, 34]. A recent meta-analysis summarized how GC in these infections delay virus clearing and extend the duration of hospitalization on average almost 10 days, without significant improvement in terms of mechanical ventilation use and risk of death [36]. As well as in MERS and SARS infections, a net benefit of GC has not been demonstrated even in other viral infections with a possible worse prognosis [37]. Previous studies found an association between GC therapy and poor clinical outcomes in patients affected by influenza [38]. Recent meta-analyses have shown that CG may almost twofold increase the risk of death in influenza pneumonia, extend the duration of intensive care unit stay by several days and increase the rate of secondary bacterial or fungal infection up to more than 3 times [39,40,41]. Indeed, the Infectious Diseases Society of America recommends against GC adjunctive therapy in patients with influenza-associated pneumonia unless clinically indicated for other reasons [42]. Clear benefits of GC treatment have not been proven even in the presence of respiratory infections by respiratory syncytial virus (RSV) in both children and adults, in which, although it has not shown to significantly affect virus shedding and immune response, it has not shown a clear improvement on clinical outcome, such as hospitalizations, either [43,44,45].
Impairment of the innate immune response against SARS-CoV-2 infection by early glucocorticoid therapy. At the top of the figure (blue part), the physiological innate immune response to viral infection (SARS-CoV-2) is summarized. Infected cells as well as macrophages and dendritic cells recognize the viral single-stranded RNA mainly through intracellular TLR7, inducing the transcription and subsequent secretion of inflammatory cytokines and type I interferon (IFN-I). They enhance antigen presentation and activate the adaptive immune system (antibody production, increased effector T-cell responses, production of type II interferon by activated T-cells and natural killer cells, etc.), counteracting viral replication. At the bottom of the figure (red part), the possible negative effects of early glucocorticoid therapy on immune response is described. Glucocorticoid therapy inhibits pro-inflammatory (IL-6 and IL-8) and antiviral (IFN-I) cytokine production and signaling pathway, decreasing the expression of the interferon-stimulated genes, suppress antigen-stimulated inflammation mediated by macrophages and dendritic cells. Glucocorticoid therapy also induces lymphopenia or can worsen a preexisting lymphopenia, hindering the T-lymphocyte immunity. Moreover, it can further downregulate membrane-bound angiotensin-converting enzyme 2 (ACE2). All these actions may contribute to viral replication and more severe lung injury. ACE2: angiotensin converting enzyme 2; TLR7: toll-like receptor 7; IFN: interferon; GC: glucocorticoid
Possible harm related to the use of glucocorticoid drugs in the early phase of SARS-CoV-2 infection: from pathophysiology to the clinical evidence
Since the beginning of the SARS-CoV-2 pandemic, several authors have discouraged the use of GC in COVID-19, based on previous evidence (increased mortality and risk of secondary infection in influenza impaired clearance of SARS-CoV and MERS-CoV and increased complications) and conflicting results of the first small clinical studies [37, 46]. Both high dosage of cumulative GC (≥ 200 mg of methylprednisolone-equivalent dose) and early initiation of GC therapy are risk factors for delayed viral clearance and may prolong viral shedding also in SARS-CoV-2 infection, although the major predictors of delayed SARS-CoV-2 clearance in moderate/severe COVID-19 were found to be an older age and a more severe disease [47,48,49], while low-dose corticosteroids probably do not have a significant impact on duration of SARS-CoV-2 viral shedding [50]. Lymphopenia might be a critical factor associated with disease severity and mortality. It is a typical characteristic of severe COVID-19, tending to persist even beyond 3 weeks after illness onset [51]. CD8 are usually present in large numbers in cellular infiltrates in the normal immune response during COVID-19 [20] and a modest lymphocytic response with small-size lymphocyte clusters was found in lung tissue from patients who died for COVID-19 [52]. GC induce lymphopenia or can worsen a preexisting lymphopenia, hindering the T-lymphocyte immunity. Early GC use is associated with lower number of CD3 + T-cells, CD8 + T-cells, and NK-cells in patients with COVID-19 pneumonia [53]. Lower levels of both CD8 T-cells and myeloid dendritic cells, together with reduced IFN response, were found in critically ill patients, who required high-flow oxygen therapy and mechanical ventilation, compared to non-critically ill patients who received low-flow oxygen therapy [54]. These aspects could at least partially explain the results of some early negative studies on GC. Early GC administration increased both the risk of mortality or mechanical ventilation usage in hospitalized patients with initial hs-CRP of less than 10 mg/dL, treated with GC within 48 h of admission [55]. Other clinical observational studies found that early initiation of GC (≤ 3 days after intensive care unit admission) was associated with an increased 90-day mortality also in critically ill patients with COVID-19 [56].
Furthermore, GC predispose to secondary bacterial and invasive fungal infections also in COVID-19 patients, as well as in influenza [57]. In cohort studies, a high incidence of bacterial superinfections and pulmonary aspergillosis were found among COVID-19 patients receiving mechanical ventilation during GC treatment [58, 59]. GC therapy is an independent risk factor for superimposed bacterial infections, especially gram-negative organisms [60]. Their incidence reported in the studies varies greatly according to studied population, setting and definition used [61]. In a cross-sectional study on 399 hospitalized COVID-19 patients, nearly half (49.6%) had a bacterial superinfection, mostly by Klebsiella and Staphylococcus Aureus, with an almost threefold increased risk for patients treated with GC [62]. In a case series by Wang et al., a significant higher incidence of pulmonary aspergillosis (9.5% vs 4.8%) was found in COVID-19 patients taking GC compared to those not taking GC [63]. Bacterial superinfections mostly consist of bloodstream infections [64] and hospital-acquired or ventilator-associated pneumonia [60, 65]. Not all studies have found this increased risk of superinfection with GC therapy [66, 67]. In the CAPE COVID Trial [68], GC treatment was not associated with an increase in the rate of secondary infections in critically ill COVID-19 patients, likely due to the low dosage of hydrocortisone used and, therefore, to its lower immunosuppressive effect.
Impaired glucose metabolism and diabetes mellitus are well-known risk factors for poor prognosis in COVID-19 and GC may worsen hyperglycemia and decompensate a known diabetes mellitus [69, 70], although not all studies are in agreement [59]. Zhang et al. reported hyperglycemia in 60% receiving corticosteroids vs 46% not receiving corticosteroids [71]. Both patients with diabetes and patients with secondary hyperglycemia showed higher inflammatory indices and longer hospital stays compared to controls [71]. Previous studies report how a new finding of hyperglycemia during COVID-19, due to the inflammatory state compounded by the GC therapy [72], is likely to be more strongly associated with a worse outcome than even pre-existing diabetes [73]. Therefore, careful hospital glucose management should be implemented in hospitalized COVID-19 patients receiving GC.
Less evidence in COVID-19 patients treated with GC is available regarding other adverse effects, such as avascular necrosis and osteoporosis. Only few case series reported avascular necrosis in the post-COVID-19 [74]. However, attention should be paid by physicians to this possible complication, especially if high and long-term GC doses have been used [75], given the close relationship previously found between osteonecrosis incidence and GC therapy in SARS patients [76] and that the same vascular damage by SARS-CoV-2 could predispose to necrosis [77,78,79]. Same considerations could be made for the risk of osteoporosis given that both single boluses of high-dose cortisone and prolonged treatments with low-dose steroids can suppress both bone formation and reabsorption, with a net loss of bone mineral mass [80].
Steroid hormones regulate multiple components of the renin–angiotensin system (RAS) [81]. Membrane-bound angiotensin-converting enzyme 2 (ACE2) is the cellular receptor for SARS-CoV-2, whose binding leads to its downregulation through its internalization and probably ACE2 shedding. ACE2 plays a very important role in lung protection, through the synthesis of angiotensin-7, exerting multiple beneficial effects (such as vasodilation, anti-hypertrophic, anti-oxidant, anti-inflammatory and anti-fibrotic actions) [8]. GC further downregulate ACE2 (Fig. 1) and recent studies found that its expression can be unregulated by INF itself [82]. It is important to note that the downregulation of membrane-bound ACE2 has a pivotal role in lung injury in COVID-19 [83]. Therefore, GC may affect COVID-19 outcome also by interfering with RAS balance.
“Cytokine Storm” and clinical evidence supporting the use of glucocorticoid drugs in COVID-19
The “cytokine storm” is a term now widely used to describe what may happen in a more advanced phase of COVID-19 that can lead to worse outcomes. However, several criticisms undermine this definition [84]. Cytokines play a key role in coordinating antimicrobial effector cells during both innate and adaptive immune responses against invasive pathogens. Therefore, a substantial increase in several cytokine levels, from IFN-γ to interleukins and chemokines, is part of the normal response to both viral and bacterial infections. There is no clear definition of "cytokine storm" associated with viral or other infections and there is no cytokine threshold (i.e., IL-1 or IL-6) known to define the presence of a "cytokine storm". Moreover, there are difficulties in the assays of cytokines both for their short half-life and for variability assays. All these factors make hard to define the limit between a physiologic inflammatory response and a “cytokine storm”, in which the immune response and its attempt to clear the pathogen can lead to a dysregulated cytokine production and inflammatory response causing cell death, coagulopathy and multiorgan dysfunction [84]. The hypothesis of favorable effects of GC and other immunomodulators, currently recommended in COVID-19 management, derives precisely from their ability to modulate cytokine levels and, therefore, limit the inflammation-mediated lung injury.
IL-6 level is a well-known lab parameter associated with worse prognosis in COVID-19 [85]. The first conflicting results of randomized controlled trials (RCTs) on anti-IL-6 monoclonal antibodies in COVID-19 [86,87,88,89,90] did not show a significant mortality benefit for treatment with tocilizumab in moderately ill hospitalized patients, while a decreased risk of mechanical ventilation has been found [91]. On the other side, tocilizumab may be more effective in patients with severe COVID-19 and high levels of systemic inflammation indices. Indeed, subsequently, the REMAP-CAP trial reported a benefit of IL-6 receptor antagonist in severely ill patients requiring organ support, with improvement in survival, reducing mortality by 8.5%, and improvement in length of stay, with earlier discharge from the intensive care unit [92]. Lately, the RECOVERY trial [93] confirmed that tocilizumab improved survival and reduced invasive mechanical ventilation need in hospitalized COVID-19 patients with hypoxia and systemic inflammation. Moreover, tocilizumab improved the chances of discharge from hospital by 28 days. Recently, a meta-analysis summarized 27 randomized trials of IL-6 receptor antagonists [94]. Although further studies are needed, the authors found significant mortality benefit limited to tocilizumab when co-administered with GC and among patients who received respiratory support with oxygen by nasal cannula, face mask, high-flow nasal oxygen or noninvasive ventilation [94, 95]. Indeed, the coadministration of GC with immunomodulatory treatments, such as IL-6 receptor antagonists but also JAK inhibitors [96] may provide additive benefits in reducing overall mortality in hospitalized patients with COVID-19 receiving supplemental oxygen therapy or ventilation [97].
The positive data coming from RCTs favoring the use of GC are mainly related to dexamethasone in severe hospitalized COVID-19 patients requiring respiratory support or supplemental oxygen, in which it was found to improve the outcome (need for invasive mechanical ventilation, ventilator-free days and death) [4, 98,99,100]. It is interesting to note that, in the RECOVERY trial, the median number of days from symptoms onset were 9 days in the “oxygen-only group” and 13 days in the “invasive mechanical ventilation group”, respectively [4], therefore, beyond what can be defined an early stage of the disease, but a more advanced phase, in which viral replication was likely declined in most patients. Moreover, dexamethasone 6 mg was associated with a reduction in mortality only among those who had symptoms for more than 7 days, while a trend for possible worse outcome was found in patients with milder and earlier disease [4]. In agreement with these findings, several retrospective observational studies found that patients affected by SARS-CoV-2 pneumonia / acute respiratory distress syndrome (ARDS) who had been treated with GC therapy started with a median time from symptom onset equal to or greater than 8–10 days, had lower in-hospital mortality than controls [101,102,103]. Inversely, starting GC before 7 days of symptom onset was not associated with lower mortality in a population of 571 hospitalized COVID-19 patients analyzed by Bahl et al. [103]. All these data suggest that GC should only be given in severe cases and several days after the onset of the disease/symptoms. However, the right time interval for GC administration is still a subject of debate and further studies are needed. In addition to the time from symptom onset, the response to GC treatment is likely related also to CRP levels and degree of lymphopenia [55, 67, 104]. Moreover, there are still doubts on the correct duration of the GC therapy (up to 10 days in the RECOVERY trial [4]), given that prolonged corticosteroid treatment may not be harmless, in terms of interference with coagulation and metabolic pathways, and long-term symptoms [105]. Finally, there are other concerns on the generalization of the RECOVERY trial data, given the several limitations of the study, regarding inclusion criteria, analyzed data and phenotyping [105]. A recent meta-analysis attempted to summarize the evidences available on the use of GC in COVID-19, highlighting the heterogeneity among the studies. Favorable effects were found in severely ill COVID-19 patients, while no benefit or even harm was found in the overall analysis [50]. Another aspect that emerges from this meta-analysis is the scarce evidence regarding the right dosage, if low-dose regimens, which seem to have no significant impact on viral shedding, or high-dose regimens are needed to obtain a clinical benefits [50]. Precisely, the low dosage/underdosing and the missing therapeutic window could perhaps explain both the lack of effect reported in some studies and the high heterogeneity in findings between them. In fact, the discrepancies in the results are more evident among the studies that used low dosages of GC [50, 98]. All this debate reflects how the role of GC therapy in COVID-19 still needs to be fully elucidated in clinical practice, particularly regarding the careful selection of the patients who could benefit from it.
Conclusion
COVID-19 likely consists of two main phases: the first one, in which viral replication peaks and a direct viral damage (mainly, but not only) to the lung is found, and the second one, in which a dysregulated inflammatory response could lead to systemic effects and worse prognosis [106]. All the evidence supporting GC administration was found in the second phase, when the dysregulated inflammatory response can contribute to organ damage. On the contrary, GC use could be harmful in the earlier phase, when the innate immunity response is fighting against viral replication. Therefore, there is neither evidence nor a rationale for the use of GC in the first phase of SARS-CoV-2 infection, especially in asymptomatic non-hospitalized patients. On the contrary, there are enough data to suggest that GC use in this phase may be harmful by promoting SARS-CoV-2 replication for longer time, increasing viral load, dysregulating the normal innate immunity with consequent possible worse lung damage. All this could contribute to increase the rates of both hospitalizations and the number of severe cases requiring respiratory support, even in younger subjects without comorbidities. In this context, the GC misuse in the first phase of SARS-CoV-2 infection should be discouraged and the indication should be restricted to selected hospitalized cases. Therefore, it is important to stress that to date, GC are recommended only in patients with severe or critical COVID-19 who require oxygen supplementation, both conventional oxygen therapy and mechanical ventilation. On the contrary, GC may be harmful and are not recommended in non-severe COVID-19, namely, outpatients who do not require additional oxygen therapy. These evidences have now been widely accepted by both international (World Health Organization [107]) and local (Italian National Institute of Health [108] and Italian Society of General Medicine [109]) recommendations on home management of COVID-19, which discourage early use of GC as “home therapy”.
References
Task force COVID-19 del Dipartimento Malattie Infettive e Servizio di Informatica, Istituto Superiore di Sanità. Epidemia COVID-19, Aggiornamento nazionale: 18 agosto 2021
Davies NG, Jarvis CI, Edmunds WJ et al (2021) Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. https://doi.org/10.1038/s41586-021-03426-1
Liu C, Ginn HM, Dejnirattisai W et al (2021) Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. https://doi.org/10.1016/j.cell.2021.06.020
Horby P, Lim WS, Emberson JR et al (2021) Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384:693–704. https://doi.org/10.1056/NEJMoa2021436
Vabret N, Britton GJ, Gruber C et al (2020) Immunology of COVID-19: current state of the science. Immunity 52:910–941. https://doi.org/10.1016/j.immuni.2020.05.002
van der Made CI, Simons A, Schuurs-Hoeijmakers J et al (2020) Presence of genetic variants among young men with severe Covid-19. JAMA 324:1–11. https://doi.org/10.1001/jama.2020.13719
Sarzani R, Giulietti F, Di PC et al (2020) Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am J Physiol 319:L325–L336
Hadjadj J, Yatim N, Barnabei L et al (2020) Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369:718–724. https://doi.org/10.1126/science.abc6027
Acharya D, Liu G, Gack MU (2020) Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol 20:397–398. https://doi.org/10.1038/s41577-020-0346-x
Zhang Q, Bastard P, Liu Z et al (2020) Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. https://doi.org/10.1126/science.abd4570
Bastard P, Rosen LB, Zhang Q et al (2020) Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. https://doi.org/10.1126/science.abd4585
Jagannathan P, Andrews JR, Bonilla H et al (2021) Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nat Commun 12:1967. https://doi.org/10.1038/s41467-021-22177-1
Grifoni A, Weiskopf D, Ramirez SI et al (2020) Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181:1489-1501.e15. https://doi.org/10.1016/j.cell.2020.05.015
Sekine T, Perez-Potti A, Rivera-Ballesteros O et al (2020) Robust T cell immunity in convalescent individuals with asymptomatic or mild Covid-19. Cell 183:158-168.e14. https://doi.org/10.1016/j.cell.2020.08.017
Rydyznski Moderbacher C, Ramirez SI, Dan JM et al (2020) Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183:996-1012.e19. https://doi.org/10.1016/j.cell.2020.09.038
Tan CW, Chia WN, Qin X et al (2020) A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 38:1073–1078. https://doi.org/10.1038/s41587-020-0631-z
Sette A, Crotty S (2021) Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184:861–880. https://doi.org/10.1016/j.cell.2021.01.007
Peng Y, Mentzer AJ, Liu G et al (2020) Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol 21:1336–1345. https://doi.org/10.1038/s41590-020-0782-6
García LF (2020) Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol 11:1441. https://doi.org/10.3389/fimmu.2020.01441
Arora K, Panda PK (2021) Steroid harms if given early in COVID-19 viraemia. BMJ Case Rep. https://doi.org/10.1136/bcr-2020-241105
Long Q-X, Liu B-Z, Deng H-J et al (2020) Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26:845–848. https://doi.org/10.1038/s41591-020-0897-1
Dispinseri S, Secchi M, Pirillo MF et al (2021) Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun 12:2670. https://doi.org/10.1038/s41467-021-22958-8
Tay MZ, Poh CM, Rénia L et al (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20:363–374. https://doi.org/10.1038/s41577-020-0311-8
Wajnberg A, Amanat F, Firpo A et al (2020) Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 370:1227–1230. https://doi.org/10.1126/science.abd7728
Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20:355–362. https://doi.org/10.1038/s41577-020-0331-4
Hoepel W, Chen H-J, Geyer CE et al (2021) High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abf8654
Shimba A, Ikuta K (2020) Control of immunity by glucocorticoids in health and disease. Semin Immunopathol 42:669–680. https://doi.org/10.1007/s00281-020-00827-8
Marcellini A, Swieboda D, Guedán A et al (2021) Glucocorticoids impair type I IFN signalling and enhance rhinovirus replication. Eur J Pharmacol 893:173839. https://doi.org/10.1016/j.ejphar.2020.173839
Flammer JR, Dobrovolna J, Kennedy MA et al (2010) The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol Cell Biol 30:4564–4574. https://doi.org/10.1128/MCB.00146-10
Thomas BJ, Porritt RA, Hertzog PJ et al (2014) Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon. Sci Rep 4:7176. https://doi.org/10.1038/srep07176
Sung JJY, Wu A, Joynt GM et al (2004) Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax 59:414–420. https://doi.org/10.1136/thx.2003.014076
Lee N, Allen Chan KC, Hui DS et al (2004) Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol Off Publ Pan Am Soc Clin Virol 31:304–309. https://doi.org/10.1016/j.jcv.2004.07.006
Arabi YM, Mandourah Y, Al-Hameed F et al (2018) Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med 197:757–767. https://doi.org/10.1164/rccm.201706-1172OC
Hui DS (2018) Systemic corticosteroid therapy may delay viral clearance in patients with Middle east respiratory syndrome coronavirus infection. Am J Respir Crit Care Med 197:700–701
Li H, Chen C, Hu F et al (2020) Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia 34:1503–1511. https://doi.org/10.1038/s41375-020-0848-3
Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet (London) 395:473–475. https://doi.org/10.1016/S0140-6736(20)30317-2
Martin-Loeches I, Torres A (2021) Corticosteroids for CAP, influenza and COVID-19: when, how and benefits or harm? Eur Respir . https://doi.org/10.1183/16000617.0346-2020
Ni Y-N, Chen G, Sun J et al (2019) The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care 23:99. https://doi.org/10.1186/s13054-019-2395-8
Yang J-W, Fan L-C, Miao X-Y et al (2015) Corticosteroids for the treatment of human infection with influenza virus: a systematic review and meta-analysis. Clin Microbiol Infect 21:956–963. https://doi.org/10.1016/j.cmi.2015.06.022
Lansbury L, Rodrigo C, Leonardi-Bee J et al (2019) Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010406.pub3
Uyeki TM, Bernstein HH, Bradley JS et al (2019) Clinical practice guidelines by the infectious diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis Am 68:e1–e47. https://doi.org/10.1093/cid/ciy866
McGee S, Hirschmann J (2008) Use of corticosteroids in treating infectious diseases. Arch Intern Med 168:1034–1046. https://doi.org/10.1001/archinte.168.10.1034
Lee FE-H, Walsh EE, Falsey AR (2011) The effect of steroid use in hospitalized adults with respiratory syncytial virus-related illness. Chest 140:1155–1161. https://doi.org/10.1378/chest.11-0047
Corneli HM, Zorc JJ, Mahajan P et al (2007) A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N Engl J Med 357:331–339. https://doi.org/10.1056/NEJMoa071255
Bartoli A, Gabrielli F, Alicandro T et al (2021) COVID-19 treatment options: a difficult journey between failed attempts and experimental drugs. Intern Emerg Med 16:281–308. https://doi.org/10.1007/s11739-020-02569-9
Cao H-R, Zhu X-Y, Zhou L et al (2021) Factors associated with delayed viral shedding in COVID-19 infected patients: a retrospective small-scale study. Respir Med. https://doi.org/10.1016/j.rmed.2021.106328
Liu J, Zhang S, Dong X et al (2020) Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest 130:6417–6428. https://doi.org/10.1172/JCI140617
Spagnuolo V, Guffanti M, Galli L et al (2020) Viral clearance after early corticosteroid treatment in patients with moderate or severe covid-19. Sci Rep 10:21291. https://doi.org/10.1038/s41598-020-78039-1
Cano EJ, Fonseca Fuentes X, Corsini Campioli C et al (2021) Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest 159:1019–1040. https://doi.org/10.1016/j.chest.2020.10.054
Wang D, Yin Y, Hu C et al (2020) Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan. China Crit Care 24:188. https://doi.org/10.1186/s13054-020-02895-6
Corredor G, Toro P, Bera K et al (2021) Computational pathology reveals unique spatial patterns of immune response in H&E images from COVID-19 autopsies: preliminary findings. J Med imaging (Bellingham, Wash) 8:17501. https://doi.org/10.1117/1.JMI.8.S1.017501
Tang X, Feng Y-M, Ni J-X et al (2021) Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with Covid-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration 100:116–126. https://doi.org/10.1159/000512063
Tiwari-Heckler S, Rauber C, Longhi MS et al (2021) Dysregulated host response in severe acute respiratory syndrome coronavirus 2-induced critical illness. Open Forum Infect Dis. https://doi.org/10.1093/ofid/ofab019
Keller MJ, Kitsis EA, Arora S et al (2020) Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19. J Hosp Med 15:489–493. https://doi.org/10.12788/jhm.3497
Li Y, Meng Q, Rao X et al (2020) Corticosteroid therapy in critically ill patients with COVID-19: a multicenter, retrospective study. Crit Care 24:698. https://doi.org/10.1186/s13054-020-03429-w
Ahmadikia K, Hashemi SJ, Khodavaisy S et al (2021) The double-edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses. https://doi.org/10.1111/myc.13256
Jeronimo CMP, Farias MEL, Val FFA et al (2021) Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 72:e373–e381. https://doi.org/10.1093/cid/ciaa1177
Yang Z, Liu J, Zhou Y et al (2020) The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect 81:e13–e20. https://doi.org/10.1016/j.jinf.2020.03.062
Nasir N, Rehman F, Omair SF (2021) Risk factors for bacterial infections in patients with moderate to severe COVID-19: a case-control study. J Med Virol 93:4564–4569. https://doi.org/10.1002/jmv.27000
Feldman C, Anderson R (2021) The role of co-infections and secondary infections in patients with COVID-19. Pneumonia (Nathan Qld) 13:5. https://doi.org/10.1186/s41479-021-00083-w
Cataño-Correa JC, Cardona-Arias JA, Porras Mancilla JP, García MT (2021) Bacterial superinfection in adults with COVID-19 hospitalized in two clinics in Medellín-Colombia, 2020. PLoS ONE. https://doi.org/10.1371/journal.pone.0254671
Wang J, Yang Q, Zhang P et al (2020) Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Crit Care 24:299. https://doi.org/10.1186/s13054-020-03046-7
Giacobbe DR, Battaglini D, Ball L et al (2020) Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest 50:e13319. https://doi.org/10.1111/eci.13319
Bartoletti M, Marconi L, Scudeller L et al (2021) Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clin Microbiol Infect 27:105–111. https://doi.org/10.1016/j.cmi.2020.09.014
Ritter LA, Britton N, Heil EL et al (2021) The impact of corticosteroids on secondary infection and mortality in critically ill Covid-19 patients. J Intensive Care Med. https://doi.org/10.1177/08850666211032175
Ho KS, Narasimhan B, Difabrizio L et al (2021) Impact of corticosteroids in hospitalised COVID-19 patients. BMJ Open Respir Res. https://doi.org/10.1136/bmjresp-2020-000766
Dequin P-F, Heming N, Meziani F et al (2020) Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with Covid-19: a randomized clinical trial. JAMA 324:1298–1306. https://doi.org/10.1001/jama.2020.16761
Smith SM, Boppana A, Traupman JA et al (2021) Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol 93:409–415. https://doi.org/10.1002/jmv.26227
Kow CS, Hasan SS (2020) Corticosteroid-related in-hospital hyperglycemia: does it negate mortality benefits in COVID-19? Clin Infect. https://doi.org/10.1093/cid/ciaa1423
Zhang Y, Li H, Zhang J et al (2020) The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: a single-centre, retrospective, observational study in Wuhan. Diabetes Obes Metab 22:1443–1454. https://doi.org/10.1111/dom.14086
Gianchandani R, Esfandiari NH, Ang L et al (2020) Managing hyperglycemia in the Covid-19 inflammatory storm. Diabetes 69:2048–2053. https://doi.org/10.2337/dbi20-0022
Fadini GP, Morieri ML, Boscari F et al (2020) Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract 168:108374. https://doi.org/10.1016/j.diabres.2020.108374
Agarwala SR, Vijayvargiya M, Pandey P (2021) Avascular necrosis as a part of “long COVID-19.” BMJ Case Rep. https://doi.org/10.1136/bcr-2021-242101
Mont MA, Pivec R, Banerjee S et al (2015) High-dose corticosteroid use and risk of hip osteonecrosis: meta-analysis and systematic literature review. J Arthroplasty 30:1506-1512.e5. https://doi.org/10.1016/j.arth.2015.03.036
Zhao R, Wang H, Wang X, Feng F (2017) Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporos Int 28:1027–1034. https://doi.org/10.1007/s00198-016-3824-z
Zhang S, Wang C, Shi L, Xue Q (2021) Beware of steroid-induced avascular necrosis of the femoral head in the treatment of Covid-19-experience and lessons from the SARS epidemic. Drug Des Devel Ther 15:983–995. https://doi.org/10.2147/DDDT.S298691
Namiranian P, Razavi SZE, Karimi M, Ayati MH (2021) Avascular necrosis in patients recovering from Covid-19. Am J Med Sci 362:331–332
Zhang B, Zhang S (2020) Corticosteroid-Induced osteonecrosis in Covid-19: a call for caution. J Bone Miner Res 35:1828–1829
Salvio G, Gianfelice C, Firmani F et al (2020) Bone metabolism in SARS-CoV-2 disease: possible osteoimmunology and gender implications. Clin Rev Bone Miner Metab. https://doi.org/10.1007/s12018-020-09274-3
Young MJ, Clyne CD, Chapman KE (2020) Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. J Endocrinol 247:R45–R62. https://doi.org/10.1530/JOE-20-0260
Busnadiego I, Fernbach S, Pohl MO et al (2020) Antiviral activity of type I, II, and III interferons counterbalances ACE2 inducibility and restricts SARS-CoV-2. MBio. https://doi.org/10.1128/mBio.01928-20
Sarzani R, Giulietti F, Di Pentima C et al (2020) Antagonizing the renin-angiotensin-aldosterone system in the era of COVID-19. Intern Emerg Med 15:885–887
Fajgenbaum DC, June CH (2020) Cytokine storm. N Engl J Med 383:2255–2273. https://doi.org/10.1056/NEJMra2026131
Moutchia J, Pokharel P, Kerri A et al (2020) Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS ONE 15:e0239802. https://doi.org/10.1371/journal.pone.0239802
Hermine O, Mariette X, Tharaux P-L et al (2021) Effect of tocilizumab vs usual care in adults hospitalized with Covid-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 181:32–40. https://doi.org/10.1001/jamainternmed.2020.6820
Stone JH, Frigault MJ, Serling-Boyd NJ et al (2020) Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 383:2333–2344. https://doi.org/10.1056/NEJMoa2028836
Rosas IO, Bräu N, Waters M et al (2021) Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 384:1503–1516. https://doi.org/10.1056/NEJMoa2028700
Veiga VC, Prats JAGG, Farias DLC et al (2021) Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 372:n84. https://doi.org/10.1136/bmj.n84
Salvarani C, Dolci G, Massari M et al (2021) Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with Covid-19 pneumonia: a randomized clinical trial. JAMA Intern Med 181:24–31. https://doi.org/10.1001/jamainternmed.2020.6615
Salama C, Han J, Yau L et al (2021) Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 384:20–30. https://doi.org/10.1056/NEJMoa2030340
Gordon AC, Mouncey PR, Al-Beidh F et al (2021) Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med 384:1491–1502. https://doi.org/10.1056/NEJMoa2100433
(2021) Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet (London) 397:1637–1645. https://doi.org/10.1016/S0140-6736(21)00676-0
Shankar-Hari M, Vale CL, Godolphin PJ et al (2021) Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 326:499–518. https://doi.org/10.1001/jama.2021.11330
Matthay MA, Luetkemeyer AF (2021) IL-6 receptor antagonist therapy for patients hospitalized for COVID-19: who, when, and how? JAMA 326:483–485
Guimarães PO, Quirk D, Furtado RH et al (2021) Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med 385:406–415. https://doi.org/10.1056/NEJMoa2101643
Stebbing J, Lauschke VM (2021) JAK inhibitors—more than just glucocorticoids. N Engl J Med 385:463–465
Sterne JAC, Murthy S, Diaz JV et al (2020) Association between administration of systemic corticosteroids and mortality among critically Ill patients With COVID-19: a meta-analysis. JAMA 324:1330–1341. https://doi.org/10.1001/jama.2020.17023
Ma S, Xu C, Liu S et al (2021) Efficacy and safety of systematic corticosteroids among severe COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. Signal Transduct Target Ther 6:83. https://doi.org/10.1038/s41392-021-00521-7
Tomazini BM, Maia IS, Cavalcanti AB et al (2020) Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 324:1307–1316. https://doi.org/10.1001/jama.2020.17021
Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A et al (2020) A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01168-20
Wu C, Hou D, Du C et al (2020) Corticosteroid therapy for coronavirus disease 2019-related acute respiratory distress syndrome: a cohort study with propensity score analysis. Crit Care 24:643. https://doi.org/10.1186/s13054-020-03340-4
Bahl A, Johnson S, Chen N-W (2021) Timing of corticosteroids impacts mortality in hospitalized COVID-19 patients. Intern Emerg Med. https://doi.org/10.1007/s11739-021-02655-6
Lu C, Liu Y, Chen B et al (2021) Prognostic value of lymphocyte count in severe COVID-19 patients with corticosteroid treatment. Signal Transduct Target Ther 6:106
Mishra GP, Mulani J (2021) Corticosteroids for COVID-19: the search for an optimum duration of therapy. Lancet Respir Med 9:e8
Cruz AF, Ruiz-Antorán B, Múñez Rubio E et al (2020) The right time for steroids In Covid-19. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa865
Keyt H (2021) WHO recommends corticosteroids for patients with severe or critical COVID-19. Ann Intern Med 174:JC2. https://doi.org/10.7326/ACPJ202101190-002
https://www.simg.it/Coronavirus/terapia_domiciliare_2303.pdf
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement. The authors report no source of funding.
Author information
Authors and Affiliations
Contributions
RS had the idea for the article. RS, FG, FS and CDP performed the literature search, the data analysis and drafted the manuscript. RS, PG and AG critically revised the work. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
The present study did not involve human participants, their data or biological material, as it is a narrative review of the published literature. Therefore, it was granted exemption from requiring ethics approval.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sarzani, R., Spannella, F., Giulietti, F. et al. Possible harm from glucocorticoid drugs misuse in the early phase of SARS-CoV-2 infection: a narrative review of the evidence. Intern Emerg Med 17, 329–338 (2022). https://doi.org/10.1007/s11739-021-02860-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-021-02860-3