Abstract
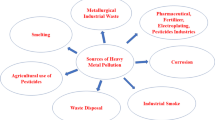
Chitosan composites and derivatives have gained wide attentions as effective biosorbents due to their low costs and high contents of amino and hydroxyl functional groups. They have showed significant potentials of removing metal ions, dyes and proteins from various media. Chemical modifications that lead to the formation of the chitosan derivatives and chitosan composites have been extensively studied and widely reported in literatures. The aims of this review were to summarize the important information of the bioactivities of chitosan, highlight the various preparation methods of chitosan-based active biosorbents, and outline its potential applications in the adsorption of heavy metal ions, dyes and proteins from wastewater and aqueous solutions.
Similar content being viewed by others
References
Ahmadi, S. J., Noori-Kalkhoran, O., and Shirvani-Arani, S., 2010. Synthesis and characterization of new ion-imprinted polymer for separation and preconcentration of uranyl (UO2 2+) ions. Journal of Hazardous Materials, 175: 193–197.
Anirudhan, T. S., Rijith, S., and Tharun, A. R., 2010. Adsorptive removal of thorium(IV) from aqueous solutions using poly(methacrylic acid)-grafted chitosan/bentonite composite matrix: Process design and equilibrium studies. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 368: 13–22.
Anirudhan, T. S., and Rijith, S., 2012. Synthesis and characterization of carboxyl terminated poly(methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium(VI) from aqueous media. Journal of Environmental Radioactivity, 106: 8–19.
Benavente, M., Moreno, L., and Martinez, J., 2011. Sorption of heavy metals from gold mining wastewater using chitosan. Journal of the Taiwan Institute of Chemical Engineers, 42: 976–988.
Bhatnagar, A., and Sillanpää, M., 2009. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater-A short review. Advances in Colloid and Interface Science, 152: 26–38.
Bhaskarapillai, A., Sevilimedu, N. V., and Sellergren, B., 2009. Synthesis and characterization of imprinted polymers for radioactive waste reduction. Industrial & Engineering Chemistry Research, 48: 3730–3737.
Bleiman, N., and Mishael, Y. G., 2010. Selenium removal from drinking water by adsorption to chitosan-clay composites and oxides: Batch and columns tests. Journal of Hazardous Materials, 183: 590–595.
Bratskaya, S. Y., Ustinov, A. Y., Azarova, Y. A., and Pestov, A. V., 2011. Thiocarbamoyl chitosan: Synthesis, characterization and sorption of Au(III), Pt(IV), and Pd(II). Carbohydrate Polymers, 85: 854–861.
Cárdenas, G., Orlando, P., and Edelio, T., 2001. Synthesis and applications of chitosan mercaptanes as heavy metal retention agent. International Journal of Biological Macromolecules, 28: 167–174.
Chang, Y. C., and Chen, D. H., 2009. Highly efficient hydrolysis of phosphodiester by a copper(II)-chelated chitosan magnetic nanocarrier. Reactive & Functional Polymers, 69: 601–605.
Chen, L. C., Kung, S. K., Chen, H. H., and Lin, S. B., 2010. Evaluation of zeta potential difference as an indicator for antibacterial strength of low molecular weight chitosan. Carbohydrate Polymers, 82: 913–919.
Chen, Y. B., Kele, M., Quinones, I., Sellergren, B., and Guiochon, G., 2001. Influence of the pH on the behavior of an imprinted polymeric stationary phase-supporting evidence for a binding site model. Journal of Chromatography A, 927: 1–17.
Chi, P., Wang, J., and Liu, C. S., 2008. Synthesis and characterization of polycationic chitosan-graft-poly (l-lysine). Materials Letters, 62: 147–150.
Claude, B., Viron-Lamy, C., Haupt, K., and Morin, P., 2010. Synthesis of a molecularly imprinted polymer for the solid-phase extraction of betulin and betulinic acid from plane bark. Phytochemical Analysis, 21: 180–185.
Crini, G., 2005. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Progress in Polymer Science, 30: 38–70.
Crini, G., 2006. Non-conventional low-cost adsorbents for dye removal: A review. Bioresource Technology, 97: 1061–1085.
Crini, G., and Badot, P. M., 2008. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solution by adsorption processes using batch studies: a review of recent literature. Progress in Polymer Science, 33: 399–447.
Dash, M., Chiellini, F., Ottenbrite, R. M., and Chiellini, E., 2011. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science, 36: 981–1014.
Dhawan, S., 2004. Chitosan microspheres as a potential carrier for drugs. International Journal of Pharmaceutics, 274: 1–33.
Dutta, P. K., Shipra Tripathi, G. K., and Mehrotra, J. D., 2009. Perspectives for chitosan based antimicrobial films in food applications. Food Chemistry, 114: 1173–1182.
Fan, L. L., Luo, C. N., Lv, Z., Lu, F. G., and Qiu, H. M., 2011. Removal of Ag+ from water environment using a novel magnetic thiourea-chitosan imprinted Ag+. Journal of Hazardous Materials, 194: 193–201.
Farzaneh, M., Mehrorang, G., Ardeshir, S., Morteza, M., and Shanaz, D., 2009. Sodium dodecyl sulfate coated poly (vinyl) chloride: An alternative support for solid phase extraction of some transition and heavy metals. Chemosphere, 74: 583–589.
Fayek, M., Horita, J., and Ripley E. M., 2011. The oxygen isotopic composition of uranium minerals: A review. Ore Geology Reviews, 41: 1–21.
Fu, G. Q., Zhao, J. C., Yu, H., Liu, L., and He, B. L., 2007. Bovine serum albumin-imprinted polymer gels prepared by graft copolymerization of acrylamide on chitosan. Reactive & Functional Polymers, 67: 442–450.
Fujiwara, K., Ramesh, A., Maki, T., Hasegawa, H., and Ueda, K., 2007. Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. Journal of Hazardous Materials, 146: 39–50.
Gan, Q., and Wang, T., 2007. Chitosan nanoparticle as protein delivery carrier-Systematic examination of fabrication conditions for efficient loading and release. Colloids and Surfaces B: Biointerfaces, 59: 24–34.
Gandhi, M. R., Kousalya, G. N., Viswanathan, N., and Meenakshi, S., 2011. Sorption behaviour of copper on chemically modified chitosan beads from aqueous solution. Carbohydrate Polymers, 83: 1082–1087.
Guibal, E., 2004. Interactions of metal ions with chitosan-based sorbents: A review. Separation and Purification Technology, 38: 43–74.
Guo, T. Y., Xia, Y. Q., Hao, G. J., Song, M. D., and Zhang, B. H., 2004. Adsorptive separation of hemoglobin by molecularly imprinted chitosan beads. Biomaterials, 25: 5905–5912.
Guo, T. Y., Xia, Y. Q., Wang, J., Song, M. D., and Zhang, B. H., 2005a. Chitosan beads as molecularly imprinted polymer matrix for selective separation of proteins. Biomaterials, 26: 5737–5745.
Guo, T. Y., Xia, Y. Q., Hao, G. J., Zhang, B. H., Fu, G. Q., Yuan, Z., He, B. L., and Kennedy, J. F., 2005b. Chemically modified chitosan beads as matrices for adsorptive separation of proteins by molecularly imprinted polymer. Carbohydrate Polymers, 62: 214–221.
Holzer, L., Münch, B., Rizzi, M., Wepf, R., Marschall, P., and Graule, T., 2010. 3D microstructure analysis of hydrated bentonite with cryo-stabilized pore water. Applied Clay Science, 47: 330–342.
Hoven, V. P., Tangpasuthadol, V., Angkitpaiboon, Y., Vallapa, N., and Kiatkamjornwong, S., 2007. Surface-charged chitosan: Preparation and protein adsorption. Carbohydrate Polymers, 68: 44–53.
Hu, X. J., Wang, J. S., Liu, Y. G., Li, X., Zeng, G. M., Bao, Z. L., Zeng, X. X., Chen, A. W., and Long, F., 2011. Adsorption of chromium(VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. Journal of Hazardous Materials, 185: 306–314.
Huang, X. D., Zou, H. F., Chen, X. M., Luo, Q. Z., and Kong, L., 2003. Molecularly imprinted monolithic stationary phases for liquid chromatographic separation of enantiomers and diastereomers. Journal of Chromatography A, 984: 273–282.
Huang, X. Y., Bin, J. P., Bu, H. T., Jiang, G. B., and Zeng, M. H., 2011. Removal of anionic dye eosin Y from aqueous solution using ethylenediamine modified chitosan. Carbohydrate Polymers, 84: 1350–1356.
Huo, H. Y., Su, H. J., and Tan, T. W., 2009. Adsorption of Ag+ by a surface molecular-imprinted biosorbent. Chemical Engineering Journal, 150: 139–144.
Juang, R. S., and Shao, H. J., 2002. A simplified equilibrium model for sorption of heavy metal ions from aqueous solutions on chitosan. Water Research, 36: 2999–3008.
Kang, Q., Zhou, W. Z., Li, Q., Gao, B. Y., Fan, J. X., and Shen, D. Z., 2009. Adsorption of anionic dyes on poly(epicholorohydrin dimethylamine) modified bentonite in single and mixed dye solutions. Applied Clay Science, 45: 280–287.
Kumar, M. N. V. R., 2000. A review of chitin and chitosan applications. Reactive & Functional Polymers, 46: 1–27.
Kumar, M., Tripathi, B. P., and Shahi, V. K., 2009. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. Journal of Hazardous Materials, 172: 1041–1048.
Kunkoro, E. P., Roussy, J., and Guibal, E., 2005. Mercury recovery by polymer-enhanced ultrafiltration: comparison of chitosan and poly(ethylenimine) used as macrolingand. Separation Science and Technology, 40: 659–684.
Kyzas, G. Z., and Lazaridis, N. K., 2009. Reactive and basic dyes removal by sorption onto chitosan derivatives. Journal of Colloid and Interface Science, 331: 32–39.
Li, Q., Yue, Q. Y., Sun, H. J., Su, Y., and Gao, B. Y., 2010. A comparative study on the properties, mechanism and process designs for the adsorption of non-ionic or anionic dyes onto cationic-polymer/bentonite. Journal of Environmental Management, 91: 1601–1611.
Li, X. L., Li, Y. F., and Ye, Z. F., 2011. Preparation of macroporous bead adsorbents based on poly(vinyl alcohol)/chitosan and their adsorption properties for heavy metals from aqueous solution. Chemical Engineering Journal, 178: 60–68.
Lin, S. B., Lin, Y. C., and Chen, H. H., 2009. Low molecular weight chitosan prepared with the aid of cellulase, lysozyme and chitinase: Characterisation and antibacterial activity. Food Chemistry, 116: 47–53.
Liu, B. J., Wang, D. F., Gao, X., Zhang, L., Xu, Y., and Li, Y. J., 2011a. Removal of arsenic from Laminaria japonica Aresch juice using As(III)-imprinted chitosan resin. European Food Research and Technology, 232: 911–917.
Liu, B. J., Wang, D. F., Li, H. Y., Xu, Y., and Zhang, L., 2011b. As(III) removal from aqueous solution using α-Fe2O3 impregnated chitosan beads with As(III) as imprinted ions. Desalination, 272: 286-292.
Liu, B. J., Wang, D. F., Xu, Y., and Huang, G. Q., 2011c. Adsorption properties of Cd(II)-imprinted chitosan resin. Journal of Materials Science, 46: 1535–1541.
Liu, Y. H., Cao, X. H., Hua, R., Wang, Y. Q., Liu, Y. T., Pang, C., and Wang, Y., 2010. Selective adsorption of uranyl ion on ion-imprinted chitosan/PVA cross-linked hydrogel. Hydrometallurgy, 104: 150–155.
Mathialagan, T., and Viraraghavan, T., 2002. Adsorption of cadmium from aqueous solutions by perlite. Journal of Hazardous Materials, B94: 291–303.
Monier, M., and El-Sokkary, A. M. A., 2010. Preparation of molecularly imprinted cross-linked chitosan/glutaraldehyde resin for enantioselective separation of l-glutamic acid. International Journal of Biological Macromolecules, 47: 207–213.
Moussavi, G., and Mahmoudi, M., 2009. Removal of azo and anthraquinone reactive dyes by using MgO nanoparticles. Journal of Hazardous Materials, 168: 806–812.
Muzzarelli, R. A. A., 1973. Natural Chelating Polymers. Pergamon Press, Oxford.
Muzzarelli, R. A. A., 2011. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydrate Polymers, 83: 1433–1445.
Nishad, P. A., Bhaskarapillai, A., Velmurugan, S., and Narasimhan, S. V., 2012. Cobalt (II) imprinted chitosan for selective removal of cobalt during nuclear reactor decontamination. Carbohydrate Polymers, 87: 2690–2696.
Popuri, S. R., Vijaya, Y., Boddu, V. M., and Abburi, K., 2009. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresource Technology, 100: 194–199.
Rachkov, A., and Minoura, N., 2001. Towards molecularly imprinted polymers selective to peptides and proteins. The epitope approach. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 1544: 255–266.
Ramesh, A., Hasegawa, H., Sugimoto, W., Maki, T., and Ueda, K., 2008. Adsorption of gold(III), platinum(W) and palladium( II) onto glycine modified crosslinked chitosan resin. Bioresource Technology, 99: 3801–3809.
Rastegarzadeh, S., Pourreza, N., and Saeedi, I., 2010. An optical chemical sensor for thorium(IV) determination based on thorin. Journal of Hazardous Materials, 173: 110–114.
Saeed, A., Fatehi, P., and Ni, Y., 2011. Chitosan as a flocculant for pre-hydrolysis liquor of kraft-based dissolving pulp production process. Carbohydrate Polymers, 86: 1630–1636.
Shameem, H., Tushar, K. G., Dabir, S. V., and Veera, M. B., 2008. Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. Journal of Hazardous Materials, 152: 826–837.
Shen, C. S., Song, S. F., Zang, L. L., Kang, X. D., Wen, Y. Z., Liu, W. P., and Fu, L. S., 2010. Efficient removal of dyes in water using chitosan microsphere supported cobalt(II) tetrasulfophthalocyanine with H2O2. Journal of Hazardous Materials, 177: 560–566.
Singh, V., Sharma, A. K., Tripathi, D. N., and Sanghi, R., 2009. Poly(methylmethacrylate) grafted chitosan: An efficient adsorbent for anionic azo dyes. Journal of Hazardous Materials, 161: 955–966.
Sinha, V. R., Singla, A. K., Wadhawan, S., Kaushik, R., Kumria, R., Bansal, K., Suntornsuk, W., Pochanavanich, P., and Suntornsuk, L., 2002. Fungal chitosan production on food processing by-products. Process Biochemistry, 37: 727–729.
Ska, D. K., 2011. Chitosan as an effective low-cost sorbent of heavy metal complexes with the polyaspartic acid. Chemical Engineering Journal, 173: 520–529.
Sobahi, T. R., Makki, M. S. I., and Abdelaal, M. Y., 2011. Carrier-mediated blends of Chitosan with polyvinyl chloride for different applications. Journal of Saudi Chemical Society, DOI: 10.1016/j.jscs.2011.03.015.
Swayampakula, K., Boddu, V. M., Nadavala, S. K., and Abburi, K., 2009. Competitive adsorption of Cu (II), Co (II) and Ni (II) from their binary and tertiary aqueous solutions using chitosan-coated perlite beads as biosorbent. Journal of Hazardous Materials, 170: 680–689.
Tang, Q., Tang, X. W., Li, Z. Z., Chen, Y. M., Kou, N. Y., and Sun, Z. F., 2009. Adsorption and desorption behavior of Pb(II) on a natural kaolin: equilibrium, kinetic and thermodynamic studies. Journal of Chemical Technology and Biotechnology, 84: 1371–1380.
Tirtom, V. N., Dinçer, A., Becerik, S., Aydemir, T., and Çelik, A., 2012. Comparative adsorption of Ni(II) and Cd(II) ions on epichlorohydrin crosslinked chitosan-clay composite beads in aqueous solution. Chemical Engineering Journal, 197: 379–386.
Varma, A. J., Deshpande, S. V., and Kennedy, J. F., 2004. Metal complexation by chitosan and its derivatives: a review. Carbohydrate Polymers, 55: 77–93.
Wan Ngah, W. S., and Isa, I. M., 1998. Comparison study of copper ion adsorption on chitosan dowex A-1 and zerolit 225. Journal of Applied Polymer Science, 67: 1067–1070.
Wan Ngah, W. S., Kamari, A., and Koay, Y. J., 2004. Equilibrium and kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. International Journal of Biological Macromolecules, 34: 155–161.
Wan Ngah, W. S., Ariff, N. F. M., Hashim, A., and Hanafiah, M. A. K. M., 2010. Malachite Green adsorption onto chitosan coated bentonite beads: Isotherms, kinetics and mechanism. Clean-Soil, Air, Water, 38: 394–400.
Wan Ngah, W. S., Teonga, L. C., and Hanafiah, M. A. K. M., 2011. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydrate Polymers, 83: 1446–1456.
Wang, J. S., Peng, R. T., Yang, J. H., Liu, Y. C., and Hu, X. J., 2011. Preparation of ethylenediamine-modified magnetic chitosan complex for adsorption of uranyl ions. Carbohydrate Polymers, 84: 1169–1175.
Wang, L., and Wang, A., 2007. Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. Journal of Hazardous Materials, 147: 979–985.
Wang, L., Xing, R. E., Liu, S., Yu, H. H., Qin, Y. K., Li, K. C., Feng, J. H., Li, R. F., and Li, P. C., 2010. Recovery of silver (I) using a thiourea-modified chitosan resin. Journal of Hazardous Materials, 180: 577–582.
Wang, X. H., Zheng, Y., and Wang, A. Q., 2009. Fast removal of copper ions from aqueous solution by chitosan-g-poly(acrylic acid)/attapulgite composites. Journal of Hazardous Materials, 168: 970–977.
Wei, J. M., Zhu, R. L., Zhu, J. X., Ge, F., Yuan, P., and He, H. P., 2009. Simultaneous sorption of crystal violet and 2-naphthol to bentonite with different CECs. Journal of Hazardous Materials, 166: 195–199.
Xiao, Y., and Zhou, X. H., 2008. Synthesis and properties of a novel crosslinked chitosan resin modified by L-lysine. Reactive & Functional Polymers, 68: 1281–1289.
Yu, C., and Mosbach, K., 2000. Influence of mobile phase composition and cross-linking density on the enantiomeric recognition properties of molecularly imprinted polymers. Journal of Chromatography A, 888: 63–72.
Zhao, Z. L., Wang, X. Q., Zhao, C., Zhu, X. G., and Du, S. Y., 2010. Adsorption and desorption of antimony acetate on sodium montmorillonite. Journal of Colloid and Interface Science, 345: 154–159.
Zhou, L. M., Liu, J. H., and Liu, Z. R., 2009. Adsorption of platinum(IV) and palladium(II) from aqueous solution by thiourea-modified chitosan microspheres. Journal of Hazardous Materials, 172: 439–446.
Zhou, L. M., Liu, Z. R., Liu, J. H., and Huang, Q. W., 2010a. Adsorption of Hg(II) from aqueous solution by ethylenediamine-modified magnetic crosslinking chitosan microspheres. Desalination, 258: 41–47.
Zhou, L. M., Xu, J. P., Liang, X. Z., and Liu, Z. R., 2010b. Adsorption of platinum(IV) and palladium(II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. Journal of Hazardous Materials, 182: 518–524.
Zhou, L. M., Jin, J. Y., Liu, Z. R., Liang, X. Z., and Shang, C., 2011. Adsorption of acid dyes from aqueous solutions by the ethylenediamine-modified magnetic chitosan nanoparticles. Journal of Hazardous Materials, 185: 1045–1052.
Zhou, Y. T., Nie, H. L., Branford-White, C., He, Z. Y., and Zhu, L. M., 2009. Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. Journal of Colloid and Interface Science, 330: 29–37.
Zhu, H. Y., Jiang, R., and Xiao, L., 2010. Adsorption of an anionic dye by chitosan/kaolin/γ-Fe2O3 composites. Applied Clay Science, 48: 522–526.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, B., Wang, D., Yu, G. et al. Adsorption of heavy metal ions, dyes and proteins by chitosan composites and derivatives — A review. J. Ocean Univ. China 12, 500–508 (2013). https://doi.org/10.1007/s11802-013-2113-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-013-2113-0