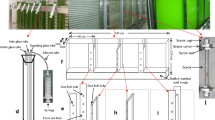
Abstract
The marine diatom Phaeodactylum tricornutum is a polymorphological, ecologically significant, and well-studied model of unicellular microalga. This diatom can accumulate diverse important metabolites. Herein, we cultured P. tricornutum in an internally installed tie-piece flat-plate photobioreactor under 14.5 m mol L−1 (high nitrogen, HN) and 2.9 m mol L−1 (low nitrogen, LN) of KNO3 and assessed its time-resolved changes in biochemical compositions. The results showed that HN was inductive to accumulate high biomass (4.1 g L−1). However, the LN condition could accelerate lipid accumulation in P. tricornutum. The maximum total lipid (TL) content under LN was up to 42.5% of biomass on day 12. Finally, neutral lipids (NLs) were 63.8% and 75.7% of TLs under HN and LN, respectively. The content of EPA ranged from 2.3% to 1.5% of dry weight during the growth period under the two culture conditions. Peak volumetric lipid productivity of 128.4 mg L−1d−1 was achieved in the HN group (on day 9). The highest volumetric productivity values of EPA, chrysolaminarin, and fucoxanthin were obtained in the exponential phase (on day 6) under HN, which were 9.6, 93.6, and 4.7 mg L−1d−1, respectively. In conclusion, extractable amounts of lipids, EPA, fucoxanthin, and chrysolaminarin could be obtained from P. tricornutum by regulating the culture conditions.
Similar content being viewed by others
References
Bai, M. D., Cheng, C. H., Wan, H. M., and Lin, Y. H., 2011. Microalgal pigments potential as byproducts in lipid production. Journal of the Taiwan Institute of Chemical Engineers, 42 (5): 783–786.
Bertrand, M., 2010. Carotenoid biosynthesis in diatoms. Photosynthesis Research, 106 (1-2): 89–102.
Borowitzka, M. A., 2013. High-value products from microalgae ? their development and commercialization. Journal of Applied Phycology, 25 (3): 743–756.
Bowler, C., Allen, A. E., Badger, J. H., Grimwood, J., Jabbari, K., Kuo, A., Maheswari, U., Martens, C., Maumus, F., Otillar, R. P., Rayko, E., Salamov, A., Vandepoele, K., Beszteri, B., Gruber, A., Heijde, M., Katinka, M., Mock, T., Valentin, K., Verret, F., Berges, J. A., Brownlee, C., Cadoret, J. P., Chiovitti, A., Choi, C. J., Coesel, S., De Martino, A., Detter, J. C., Durkin, C., Falciatore, A., Fournet, J., Haruta, M., Huysman, M. J., Jenkins, B. D., Jiroutova, K., Jorgensen, R. E., Joubert, Y., Kaplan, A., Kroger, N., Kroth, P. G., La Roche, J., Lindquist, E., Lommer, M., Martin-Jezequel, V., Lopez, P. J., Lucas, S., Mangogna, M., McGinnis, K., Medlin, L. K., Montsant, A., Oudot-Le Secq, M. P., Napoli, C., Obornik, M., Parker, M. S., Petit, J. L., Porcel, B. M., Poulsen, N., Robison, M., Rychlewski, L., Rynearson, T. A., Schmutz, J., Shapiro, H., Siaut, M., Stanley, M., Sussman, M. R., Taylor, A. R., Vardi, A., von Dassow, P., Vyverman, W., Willis, A., Wyrwicz, L. S., Rokhsar, D. S., Weissenbach, J., Armbrust, E. V., Green, B. R., Van De Peer, Y., and Grigoriev, I. V., 2008. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature, 456 (7219): 239–244.
Brennan, L., and Owende, P., 2010. Biofuels from microalgae ? A review of technologies for production, processing, and extractions of biofuels and co-products. Renewable and Sustainable Energy Reviews, 14 (2): 557–577.
Breuer, G., Lamers, P. P., Martens, D. E., Draaisma, R. B., and Wijffels, R. H., 2012. The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresource Technology, 124: 217–226.
Brussaard, C. P. D., Noordeloos, A. A. M., and Riegman, R., 1997. Autolysis kinetics of the marine diatom Ditylum brightellii (Bacillariophyceae) under nitrogen and phosporus limitation and starvation. Journal of Phycology, 33: 980–987.
Carreto, J. I., and Catoggio, J. A., 1976. Variations in pigment contents of the diatom Phaeodactylum tricornutum during growth. Marine Biology, 36 (2): 105–112.
Chauton, M. S., Reitan, K. I., Norsker, N. H., Tveterås, R., and Kleivdal, H. T., 2015. A techno-economic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: research challenges and possibilities. Aquaculture, 436: 95–103.
Chisti, Y., 2007. Biodiesel from microalgae. Biotechnology Advances, 25 (3): 294–306.
Cohen, Z., Norman, H. A., and Heimer, Y. M., 1993. Potential use of substituted pyridazinones for selecting polyunsaturated fatty acid overproduing cell lines of algae. Phytochemistry, 32: 259–264.
Dubios, M., Gillies, K. A., Hamilton, J. K., Rebers, P. A. T., and Smith, F., 1956. Colorimetric method for the determination of sugars and related substances. Analytical Chemistry, 28: 350–356.
Gao, B., Yang, J., Lei, X., Xia, S., Li, A., and Zhang, C., 2016. Characterization of cell structural change, growth, lipid accumulation, and pigment profile of a novel oleaginous microalga, Vischeria stellata (Eustigmatophyceae), cultured with different initial nitrate supplies. Journal of Applied Phycology, 28 (2): 821–830.
Gong, Y., Guo, X., Wan, X., Liang, Z., and Jiang, M., 2013. Triacylglycerol accumulation and change in fatty acid content of four marine oleaginous microalgae under nutrient limitation and at different culture ages. Journal of Basic Microbiology, 53 (1): 29–36.
Granum, E., and Myklestad, S. M., 2001. Mobilization of ß-1, 3-glucan and biosynthesis of amino acids induced by NH4+ addition to N-limited cells of the marine diatom Skeletonema costatum (Bacillariophyceae). Journal of Phycology, 37 (5): 772–782.
Granum, E., and Myklestad, S. M., 2002. A simple combined method for determination of ß-1, 3-glucan and cell wall polysaccharides in diatoms. Hydrobiologia, 477: 155–161.
Griffiths, M. J., and Harrison, S. T. L., 2009. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Journal of Applied Phycology, 276: 23–25.
Grima, E. M., Pé rez, J. A. S., Camacho, F. G., Sá nchez, J. G., and Alonso, D. L., 1993. n-3 PUFA productivity in chemostat cultures of microalgae. Applied Microbiology and Biotechnology, 38 (5): 599–605.
Guedes, A. C., Meireles, L. A., Amaro, H. M., and Malcata, F. X., 2010. Changes in lipid class and fatty acid composition of cultures of Pavlova lutheri, in response to light intensity. Journal of the American Oil Chemists Society, 87 (7): 791–801.
Guschina, I. A., and Harwood, J. L., 2009. Algal lipids and effect of the environment on their biochemistry. In: Lipids in Aquatic Ecosystems. Arts, M. T., et al., eds., Springer, New York, 1–25.
Harisko, I., and Posten, C., 2014. Biorefinery of microalgaeopportunities and constraints for different production scenarios. Biotechnology, 9: 739–752.
Hayward, J., 1968. Studies on the growth of Phaeodactylum tricornutum IV.Comparison of different isolates. Journal of the Marine Biological Association of the United Kingdom, 48 (03): 657–666.
Hodgson, P. A., Henderson, R. J., Sargent, J. R., and Leftley, J. W., 1991. Patterns of variation in the lipid class and fatty acid composition of Nannochloropsis oculata (Eustigmatophyceae) during batch culture. Journal of Applied Phycology, 3 (2): 169–181.
Ibañez, E., Herrero, M., Mendiola, J. A., and Castro-Puyana, M., 2012. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates. In: Marine Bioactive Compounds. Hayes, M., ed., Springer, New York, 55–98.
Khozin-Goldberg, I., Iskandarov, U., and Cohen, Z., 2011. LCPUFA from photosynthetic microalgae: Occurrence, biosynthesis, and prospects in biotechnology. Applied Microbiology and Biotechnology, 91 (4): 905–915.
Khozin-Goldberg, I., Shrestha, P., and Cohen, Z., 2005. Mobilization of arachidonyl moieties from triacylglycerols into chloroplastic lipids following recovery from nitrogen starvation of the microalga Parietochloris incise. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1738 (1): 63–71.
Kim, J. C., 2014. Solvent extraction of fucoxanthin from Phaeodactylum tricornutum. Separation Science and Technology, 49: 410–415.
Kim, S. M., Jung, Y. J., Kwon, O. N., Cha, K. H., Um, B. Y., Chung, D., and Pan, C. H., 2012. A potential commercial source of fucoxanthin extracted from the microalga Phaedactylum tricornutum. Applied Biochemistry and Biotechnology, 166: 1843–1855.
Kusaikin, M. I., Ermakova, S. P., Shevchenko, N. M., Isakov, V. V., Gorshkov, A. G., Vereshchagin, A. L., Grachev, M. A., and Zvyagintseva, T. N., 2010. Structural characteristics and antitumor activity of a new chrysolaminaran from the diatom alga Synedra acus. Chemistry of Natural Compounds, 46 (1): 1–4.
Larson, T. R., and Rees, T. A. V., 1996. Changes in cell composition and lipid metabolism mediated by sodium and nitrogen availability in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). Journal of Phycology, 32 (3): 388–393.
Lebeau, T., and Robert, J. M., 2003. Diatom cultivation and biotechnologically relevant products. Part I: Cultivation at various scales. Applied Microbiology and Biotechnology, 60 (6): 612–623.
Liang, Y., Beardall, J., and Heraud, P., 2006. Changes in growth, chlorophyll fluorescence and fatty acid composition with culture age in batch cultures of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). Botanica Marina, 49 (2): 165–173.
Mann, J. E., and Myers, J., 1968. On pigments, growth, and photosynthesis of Phaeodactylum tricornutum. Journal of Phycology, 4 (4): 349–355.
Medina, A. R., Grima, E. M., Giménez, A. G., and González, M. I., 1998. Downstream processing of algal polyunsaturated fatty acids. Biotechnology Advances, 16 (3): 517–580.
Meng, X., Yang, J., Xu, X., Zhang, L., Nie, Q., and Xian, M., 2009. Biodiesel production from oleaginous microorganisms. Renewable Energy, 34 (1): 1–5.
Molina, G. E., Belarbi, E. H., Acién, F. F. G., Medina, A. R., and Chisti, Y., 2003. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnology Advances, 20 (7): 491–515.
Muramatsu, D., Iwai, A., Aoki, S., Uchiyama, H., Kawata, K., Nakayama, Y., Nikawa, Y., Kusano, K., Okabe, M., and Miyazaki, T., 2012. ß-Glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PloS One, 7 (7): e41399.
Nagao, K., and Yanagita, T., 2005. Conjugated fatty acids in food and their health benefits. Journal of Bioscience and Bioengineering, 100 (2): 152–157.
Peng, J., Yuan, J. P., Wu, C. F., and Wang, J. H., 2011. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Marine Drugs, 9 (10): 1806–1828.
Prommuak, C., Pavasant, P., Quitain, A. T., Goto, M., and Shotipruk, A., 2013. Simultaneous production of biodiesel and free lutein from Chlorella vulgaris. Chemical Engineering & Technology, 36 (5): 733–739.
Reis, A., Gouveia, L., Veloso, V., Fernandes, H. L., Empis, J., and Novais, J. M., 1996. Eicosapentaenoic acid-rich biomass production by the microalga Phaeodactylum tricornutum in a continuous-flow reactor. Bioresource Technology, 55 (1): 83–88.
Silva, B. A. M., Torzillo, G., Kopecký, J., and Masojídek, J., 2013. Productivity and biochemical composition of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown outdoors in tubular photobioreactors and open ponds. Biomass and Bioenergy, 54: 115–122.
Størseth, T. R., Kirkvold, S., Skjermo, J., and Reitan, K. I., 2006. A branched β-d-(1→3, 1→6)-glucan from the marine diatom Chaetoceros debilis (Bacillariophyceae) characterized by NMR. Carbohydrate Research, 341 (12): 2108–2114.
Tonon, T., Harvey, D., Larson, T. R., and Graham, I. A., 2002. Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochemistry, 61 (1): 15–24.
Ugwu, C. U., Aoyagi, H., and Uchiyama, H., 2008. Photobioreactors for mass cultivation of algae. Bioresource Technology, 99 (10): 4021–4028.
Wen, Z. Y., and Chen, F., 2003. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnology Advances, 21 (4): 273–294.
Widjaja, A., Chien, C., and Ju, Y., 2009. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. Journal of the Taiwan Institute of Chemical Engineers, 40 (1): 13–20.
Xia, S., Gao, B., Li, A., Xiong, J., Ao, Z., and Zhang, C., 2014. Preliminary characterization, antioxidant properties and production of chrysolaminarin from marine diatom Odontella aurita. Marine Drugs, 12 (9): 4883–4897.
Xia, S., Wan, L., Li, A., Sang, M., and Zhang, C., 2013a. Effects of nutrients and light intensity on the growth and biochemical composition of a marine microalga Odontella aurita. Chinese Journal of Oceanology and Limnology, 31: 1163–1173.
Xia, S., Wang, K., Wan, L., Li, A., Hu, Q., and Zhang, C., 2013b. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Marine Drugs, 11 (7): 2667–2681.
Yang, X., Wang, R., Zhang, S., Zhu, W., Tang, J., Liu, J., Chen, P., Zhang, D., Ye, W., and Zheng, Y., 2014. Polysaccharides from Panax japonicus CA Meyer and their antioxidant activities. Carbohydrate Polymers, 101: 386–391.
Yongmanitchai, W., and Ward, O. P., 1991. Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Applied and Environmental Microbiology, 57 (2): 419–425.
Zhang, C., Zmora, O., Kopel, R., and Richmond, A., 2001. An industrial-size flat plate glass reactor for mass production of Nannochloropsi sp. (Eustigmatophyceae). Aquaculture, 195 (1): 35–49.
Zhang, Z., Wang, X., Mo, X., and Qi, H., 2013. Degradation and the antioxidant activity of polysaccharide from Enteromorpha linza. Carbohydrate Polymers, 92 (2): 2084–2087.
Acknowledgement
This work was supported by the following fundings: the Natural Science Foundation of China (No. 31170337); the National High Technology Research and Development Program of China (863 Program) (No. 2013AA 065805); the National Basic Research Program of China (973 Program) (No. 2011CB2009001); the Special Program for Low-Carbon, Reform and Development Commission of Guangdong Province (No. 2011-051).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, B., Chen, A., Zhang, W. et al. Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions. J. Ocean Univ. China 16, 916–924 (2017). https://doi.org/10.1007/s11802-017-3174-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-017-3174-2