Abstract
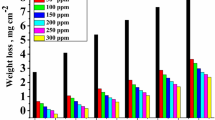
We investigated the absorption ability of potassium salts of amino acid solutions for carbon dioxide and compared the results with MEA. The corrosion and degradation behavior were investigated in a CO2 absorption process using aqueous potassium salts of glycine and taurine. The experimental parameters varied were the concentration, amino acid type, temperature, CO2 loading, piperazine, and the presence of corrosion inhibitors. The corrosion characteristics of carbon steel were measured with potassium glycinate and potassium taurate solutions over a wide range of concentrations (1.5 to 5.0 M) and temperatures (313.15 to 353.15 K). The corrosion rate was calculated using a weight loss method averaging the results of four specimens. The experimental results indicate that increases in the concentration of the aqueous amino acid salts, solution temperature, CO2 loading, and piperazine concentration accelerate the corrosion rate. In addition, corrosion inhibitors were proven to be effective in controlling corrosion.
Similar content being viewed by others
References
A. F. Portugal, P. W. J. Derks, G. F. Versteeg, F.D. Magalhaes and A. Mendes, Chem. Eng. Sci., 62(23), 6534 (2007).
S. Lee, J.W. Park, H. J. Song, S. Maken and T. Filburn, Energy Policy, 36(1), 326 (2008).
S. Lee, H. J. Song, S. Maken, H. C. Shin, H. C. Song and J.W. Park, J. Chem. Eng. Data, 51(2), 504 (2006).
S. Lee, S. Choi, S. Maken, H. J. Song, H. C. Shin and J.W. Park, J. Chem. Eng. Data, 50(5), 1773 (2005).
P. S. Kumar, J.A. Hogendoorn, G. F. Versteeg and P.H. M. Feron, AIChE J., 49(1), 203 (2003).
H. J. Song, S. Lee, S. Maken, J. J. Park and J.W. Park, Fluid Phase Equilibria, 246(1–2), 1 (2006).
S. Lee, H. J. Song, S. Maken, S.K. Yoo and J.W. Park, Korean J. Chem. Eng., 25(1), 1 (2008).
J. Van Holst, S. R. A. Kersten and K. J.A. Hogendoorn, J. Chem. Eng. Data, 53(6), 1286 (2008).
J. Zhang, S. Zhang, K. Dong, Y. Zhang, Y. Shen and X. Lv, Chemistry — A European Journal, 12(15), 4021 (2006).
P. S. Kumar, J.A. Hogendoorn, P.H. M. Feron and G. F. Versteeg, Ind. Eng. Chem. Res., 42(12), 2832 (2003).
P. S. Kumar, J.A. Hogendeeorn, S. J. Timmer, P.H. M. Feron and G. F. Versteeg, Ind. Eng. Chem. Res., 42(12), 2841 (2003).
M. S. DuPart, T. R. Bacon and D. J. Edwards, Hydrocarbon Processing, 72(5), 89 (1993).
E.N. Hawkes and B. F. Mago, Hydrocarbon Processing, 50(8), 109 (1971).
S. Lee, S. Maken, J.W. Park, H. J. Song, J. J. Park, J.G. Shim, J. H. Kim and H. M. Eum, Fuel, 87(8–9), 1734 (2008).
A. Veawab, P. Tontiwachwuthikul and A. Chakma, Ind. Eng. Chem. Res., 38(1), 310 (1999).
S. Ma’mun, H. F. Svendsen, K.A. Hoff and O. Juliussen, Energy Conver. Manage., 48(1), 251 (2007).
I. R. Soosaiprakasam and A. Veawab, International J. Greenhouse Gas Control, 2(4), 553 (2008).
Chemical Compositions of SAE Carbon Steels, http://www.kspipe.com/datacenter-6.htm.
M. S. DuPart, T. R. Bacon and D. J. Edwards, Hydrocarbon Processing, 72(4), 75 (1993).
A. Veawab, P. Tontiwachwuthikul and A. Chakma, Ind. Eng. Chem. Res., 38(10), 3917 (1999).
A. Veawab, P. Tontiwachwuthikul and S. D. Bhole, Chem. Eng. Commun., 144, 65 (1996).
A. Veawab, P. Tontiwachwuthikul and S. D. Bhole, Ind. Eng. Chem. Res., 36(1), 264 (1997).
D.M. Austgen, G. T. Rochelle, P. Xiao and C. C. Chen, Ind. Eng. Chem. Res., 28(7), 1060 (1989).
S. Lee, H. J. Song, S. Maken and J.W. Park, Ind. Eng. Chem. Res., 46(5), 1578 (2007).
M. Nainar and A. Veawab, Energy Procedia, 1(1), 231 (2009).
J. T. Cullinane and G. R. Rochelle, Chem. Eng. Sci., 59(17), 3619 (2004).
L. Rob, The promoter effect of piperazine on the removal of carbon dioxide, 7th January (2004).
J. Oexmann, C. Hensel and A. Kather, International J. Greenhouse Gas Control, 2(4), 539 (2008).
A. Veawab, P. Tontiwachwuthikul and A. Chakma, Ind. Eng. Chem. Res., 40(22), 4771 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahn, S., Song, HJ., Park, JW. et al. Characterization of metal corrosion by aqueous amino acid salts for the capture of CO2 . Korean J. Chem. Eng. 27, 1576–1580 (2010). https://doi.org/10.1007/s11814-010-0246-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0246-z