Abstract
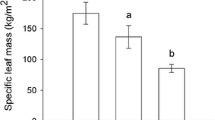
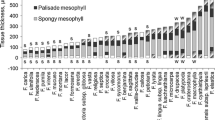
This study determined the temporal patterns of herbivory on Handroanthus ochraceus (Cham.) Mattos (Bignoniaceae) along a successional gradient in a seasonally dry tropical forest (SDTF) in southeastern Brazil. We assessed the diversity of free-feeding herbivore insects (sap-suckers and leaf-chewers), leaf herbivory rates, leaf nitrogen content, phenolic compounds, and spider abundance through the rainy season in three different successional stages (early, intermediate, and late). Sampling was conducted in December, at the beginning of the rainy season (with fully expanded young leaves), February (mid-aged leaves), and April, at the end of rainy season (old leaves). Fifteen reproductive trees of H. ochraceus were sampled per successional stage in each month of sampling. Herbivore diversity was highest in the early stage of succession, but herbivory rates were highest in the intermediate and late stages. This result was probably related to differences in herbivore community composition and leaf quality across successional stages. The highest herbivore abundance was found in April in the early successional stage. In addition, we found low levels of herbivory in the intermediate and late successional stages in the second half of the rainy season. For each successional stage, leaf nitrogen content decreased through the rainy season, whereas the concentration of phenolic compounds increased. For the intermediate and late successional stages, the temporal changes that took place as the rainy season progressed corroborated the following hypotheses postulated for SDTFs: (1) both the abundance of chewing insects and herbivory rates decreased, (2) the abundance of natural enemies (i.e., spiders) increased, and (3) leaf quality decreased. These results suggest that the described herbivory patterns are robust for advanced successional stages (intermediate and late) of the SDTFs, but may not apply to early successional stages of these forests.







Similar content being viewed by others
References
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell Scientific Publications, Oxford
Antunes FZ (1994) Caracterização climática–caatinga do estado de minas gerais. Info Agro 17:15–19
Barret MA, Stiling P (2007) Relationships among key deer, insect herbivores and plant quality. Ecol Res 22:268–273
Basset Y, Aberlenc HP, Barrios H, Curletti G, Berenger JM, Vesco JP, Causse P, Haug A, Hennion AS, Lesobre L, Marques F, O’Meara R (2001) Stratification and diel activity of arthropods in a lowland rainforest in Gabon biological. J Linn Soc 72:585–607
Basset Y, Novotny V, Miller SE, Kitching RL (2003) Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge
Boege K (2004) Induced responses in three tropical dry forest plant species–direct and indirect effects on herbivory. Oikos 107:541–548
Boege K (2005) Herbivore attack in Casearia nitida influenced by plant ontogenetic variation in foliage quality and plant architecture. Oecologia 143:117–125
Borror DJ, Triplehorn CA, Johnson NF (2002) An introduction to the study of insects. Saunders College Publishing, New York
Brown BJ, Ewel JJ (1987) Herbivory in complex and simple tropical successional ecosystems. Ecology 68:108–116
Bryant JP, Chapin FS III, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Campos RL, Vasconcelos HL, Ribeiro SP, Neves FS, Soares JP (2006) Relationship between tree size and insect assemblages associated with Anadenanthera macrocarpa. Ecography 29:442–450
Cates RG, Orians GH (1975) Successional status and the palatability of plants to generalized herbivores. Ecology 56:410–418
Close DC, McArthur C (2002) Rethinking the role of many plant phenolics–protection from photodamage not herbivores? Oikos 199:166–172
Coley PD, Barone JA (1996) Herbivory and plant defenses in tropical forests. Annu Rev Ecol Syst 27:305–335
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant anti-herbivore defense. Science 230:895–899
Cornelissen TG, Fernandes GW (2001) Induced defences in the neotropical tree Bauhinia brevipes (Vog.) to herbivory: effects of damage-induced changes on leaf quality and insect attack. Tree 15:236–241
Crawley M (2002) Statistical computing: an introduction to data analysis using S-Plus. Wiley, London
Davidson DW (1993) The effects of herbivory and granivory on terrestrial plant succession. Oikos 68:23–35
Didham RK, Springate ND (2003) Determinants of temporal variation in community structure. In: Basset Y, Novotny V, Miller S, Kitching R (eds) Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge, pp 28–39
Dirzo R, Boege K (2008) Patterns of herbivory and defense in tropical dry and rain forests. In: Carson W, Schnitzer SA (eds) Tropical forest community ecology. Blackwell Science, West Sussex, pp 63–78
Dirzo R, Domínguez CA (1995) Plant-herbivore interactions in Mesoamerican tropical dry forest. In: Bullock SH, Mooney A, Medina E (eds) Seasonally dry tropical forest. Cambridge University Press, Cambridge, pp 304–309
Ernest KA (1989) Insect herbivory on a tropical understory tree: effects of leaf age and habitat. Biotropica 21:194–199
Fernandes GW, Castro FMC, Faria ML, Marques ESA, Greco MKB (2004) Effects of hygrothermal stress, plant richness, and architecture on mining insect diversity. Biotropica 36:240–247
Filip V, Dirzo RJ, Maass M, Sarukhán J (1995) Within- and among-year variation in the levels of herbivory on the foliage of trees from a Mexican tropical deciduous forest. Biotropica 27:78–86
Gentry AH (1992) Bignoniaceae–part II (Tribe Tecomeae). Flora neotropica, monograph 25(II). New York Botanical Garden, New York
Grose SO, Olmstead RG (2007) Taxonomic revisions in the polyphyletic genus Tabebuia s.l. (Bignoniaceae). Syst Bot 32:660–670
Gruner DS, Polhemus DA (2003) Arthropod assemblages across a long chronosequence in the Hawaiian islands. In: Basset Y, Novotny V, Miller S, Kitching R (eds) Arthropods of tropical forests: Spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge, pp 135–145
Hagerman AE (1987) Radial diffusion method for determining tannin in plant extracts. J Chem Ecol 13:437–449
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
IEF—Instituto Estadual de Florestas (2000) Parecer técnico para a criação do Parque Estadual da Mata Seca. Relatório técnico, Belo Horizonte
Janzen DH (1981) Patterns of herbivory in a tropical deciduous forest. Biotropica 13:271–282
Janzen DH, Waterman PG (1984) A seasonal census of phenolics, fibre and alkaloids in foliage of forest trees in Costa Rica: some factors influencing their distribution and relation to host selection by Sphingidae and Saturniidae. Biol J Linn Soc 21:439–454
Kalácska M, Sanchez-Azofeifa GA, Calvo-Alvarado JC, Quesada M, Rivard B, Janzen DH (2004) Species composition, similarity and diversity in three successional stages of seasonally dry tropical forest. For Ecol Manage 200:227–247
Leite LO, Borges MAZ, Lima CA, Gonçalves RMM, Siqueira PR (2008) Variação espaço-temporal do uso de recursos pela avifauna do Parque Estadual da Mata Seca. MG Biota 1:54–60
Lewinsohn TM, Novotny V, Basset Y (2005) Insects on plants: diversity of herbivore assemblages revisited. Annu Rev Ecol Syst 36:597–620
Ludwig D, Walter B, Holling CS (1997) Sustainability, stability, and resilience. Conserv Ecol 1:1–27
Madeira BG, Espírito-Santo MM, DÂngelo-Neto S, Nunes YRF, Sánchez-Azofeifa GA, Fernandes GW, Quesada M (2009) Changes in tree and liana communities along a successional gradient in a tropical dry forest in south-eastern Brazil. Plant Ecol 291:291–304
Mattson JMJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Mendonça MS (2001) Galling insect diversity: the resource synchronization hypothesis. Oikos 95:171–176
Moran CV, Southwood TRE (1982) The guild composition of arthropod communities in trees. J Anim Ecol 51:289–306
Moreira PA, Fernandes GW, Collevatti RG (2009) Fragmentation and spatial genetic structure in Tabebuia ochracea (Bignoniaceae), a seasonally dry Neotropical tree. For Ecol Manage 258:2690–2695
Neves FS, Araújo LS, Fagundes M, Espírito-Santo MM, Fernandes GW, Sánchez-Azofeifa GA, Quesada M (2010a) Canopy herbivory and insect herbivore diversity in a dry forest-savanna transition in Brazil. Biotropica 42:112–118
Neves FS, Braga RF, Espírito-Santo MM, Delabie JHC, Fernandes GW, Sánchez-Azofeifa GA (2010b) Diversity of arboreal ants in a Brazilian tropical dry forest: effects of seasonality and successional stage. Sociobiology 56:177–194
Novotny V, Basset Y (1998) Seasonality of sap-sucking insects (Auchenorrhyncha, Hemiptera) feeding on Ficus (Moraceae) in a lowland rain forest in New Guinea. Oecologia 115:514–522
Novotny V, Basset Y, Kitching R (2003) Herbivore assemblages and their food resources. In: Basset Y, Novotny V, Miller S, Kitching R (eds) Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge, pp 40–53
Pezzini FF (2008) Fenologia e características reprodutivas em comunidades arbóreas de três estágios sucessionais em Floresta Estacional Decidual do norte de Minas Gerais. Master dissertation, Universidade Federal de Minas Gerais, Belo Horizonte
Pezzini FF, Brandão D, Ranieri BD, Espírito-Santo MM, Jacobi CM, Fernandes GW (2008) Polinização, dispersão de sementes e fenologia de espécies arbóreas no Parque Estadual da Mata Seca. MG Biota 1:37–45
Poorter L, Plassche MV, Willems S, Boot RGA (2004) Leaf traits and herbivory rates of tropical tree species differing in successional status. Plant Biol 6:746–754
Price P (1997) Insect ecology. Wiley, New York
Quesada L, Sanchez-Azofeifa GA, Alvarez-Añorve M, Stoner KE, Avila-Cabadilla L, Calvo-Alvarado J, Castillo J, Espírito-Santo MM, Fagundes M, Fernandes GW, Gamonb J, Lopezaraiza-Mikel M, Lawrence D, Morellato LPC, Powers JS, Neves FS, Rosas-Guerrero V, Sayago R, Sanchez-Montoya G (2009) Succession and management of tropical dry forests in the Americas: review and new perspectives. For Ecol Manage 258:1014–1024
R Development Core Team (2009) R: a language and environment for statistical computing. R foundation for statistical computing, http://www.r-project.org. Accessed 21 July 2009
Rasband WS (2006) ImageJ, US. National Institutes of Health, Bethesda, Maryland, http://rsb.info.nih.gov/ij. Accessed 15 July 2009
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH (eds) Herbivores: their interaction with secondary plant metabolites. Academic Press, London, pp 3–54
Ribeiro SP (1998) The role of herbivory in Tabebuia spp. life history and evolution. Ph.D Dissertation, Imperial College at Silwood Park, London
Ribeiro SP, Brown VK (1999) Insect herbivory within tree crowns of Tabebuia aurea and T. ochracea (Bignoniaceae): contrasting the Brazilian Cerrado with the wetland ‘Pantanal Matogrossense’. Selbyana 120:159–170
Ribeiro SP, Brown V (2006) Prevalence of monodominant vigorous tree populations in the tropics: herbivory pressure on Tabebuia species in very different habitats. J Ecol 94:932–941
Ribeiro SP, Pimenta HR (1991) Padrões de abundância e de distribuição temporal de herbívoros de vida livre em Tabebuia ochracea (Bignoniaceae). Ann Soc Entomol Brasil 20:428–448
Ribeiro SP, Pimenta HP, Fernandes GW (1994) Herbivory by chewing and sap-feeding insects on Tabebuia ochracea. Biotropica 26:302–307
Sanders D, Nickel H, Grützner T, Platner C (2008) Habitat structure mediates top–down effects of spiders and ants on herbivores. Basic Appl Ecol 9:152–160
Santos JC, Almeida-Cortez JS, Fernandes GW (2011) Diversity of gall-inducing insects in the high altitude wetland forests in Pernambuco, Northeastern Brazil. Braz J Biol 71:47–56
Siemann E, Haarstad J, Tilman D (1999) Dynamics of plant and arthropod diversity during old field succession. Ecograph 22:406–414
Silva JO, Jesus FM, Fagundes M, Fernandes GW (2009) Esclerofilia, taninos e insetos herbívoros associados a Copaifera langsdorffii Desf. (Fabaceae: Caesalpinioideae) em área de transição Cerrado-Caatinga no Brasil. Ecol Austral 19:197–206
Silva JO, Oliveira KN, Santos KJ, Espírito-Santo MM, Neves FS, Faria ML (2010) Efeito da estrutura da paisagem e do genótipo de Eucalyptus na abundância e controle biológico de Glycaspis brimblecombei Moore (Hemiptera: Psyllidae). Neotrop Entomol 39:91–96
Stanton N (1975) Herbivore pressure on two types of tropical forests. Biotropica 7:8–11
Stiling P, Moon DC (2005) Quality or quantity: the direct and indirect effects of host plants on herbivores and their natural enemies. Oecologia 142:413–420
Strong DR, Lawton JH, Southwood TRE (1984) Insects on plants: community patterns and mechanisms. Blackwell Scientific Publication, London
Sullivan JJ (2000) How the sapling specialist shoot-borer, Cromarcha stroudagnesia (Lepidoptera, Pyralidae, Chrysauginae), alters the population dynamics of the Costa Rican tropical dry forest tree Tabebuia ochracea (Bignoniaceae). Ph.D Dissertation, University of Pennsylvania, Philadelphia
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. J Sci Food Agric 10:63–68
Swanson ME, Franklin JF, Beschta RL, Crisafulli CM, DellaSala DA, Hutto RL, Lindenmayer DB, Swanson FJ (2011) The forgotten stage of forest succession: early-successional ecosystems on forest sites. Front Ecol Environ 9:117–125
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, Oxford
Varanda EM, Pais MP (2006) Insect folivory in Didymopanax vinosum (Apiaceae) in a vegetation mosaic of Brazilian Cerrado. Braz J Biol 66:671–680
Wolda H (1988) Insect seasonality, why? Ann Rev Ecol Syst 19:1–18
Acknowledgments
We are very grateful to M Fagundes, SP Ribeiro and FS Neves for their valuable comments on the early versions of this manuscript. We also thank RA Andrade, KN Oliveira, SF Magalhães and A Mendes for field assistance, and SP Ribeiro for insect identification. Logistical support was provided by the Instituto Estadual de Florestas (IEF), and the financial support was provided by Conselho Nacional de Pesquisa—CNPq (474508-07), Fundação de Amparo à Pesquisa de Minas Gerais-FAPEMIG (CRA-2288/07 and CRA APQ-3042-5.03/07), and the Inter-American Institute for Global Change Research (IAI-CRN II-021). We gratefully acknowledge FAPEMIG for a MSc scholarship to JO Silva and a research scholarship to MM Espírito-Santo. This study was in partial fulfillment of requirements for the Master degree at Universidade Estadual de Montes Claros.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Gimme Walter.
Appendix
Appendix
See Table 3.
Rights and permissions
About this article
Cite this article
Silva, J.O., Espírito-Santo, M.M. & Melo, G.A. Herbivory on Handroanthus ochraceus (Bignoniaceae) along a successional gradient in a tropical dry forest. Arthropod-Plant Interactions 6, 45–57 (2012). https://doi.org/10.1007/s11829-011-9160-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-011-9160-5