Abstract
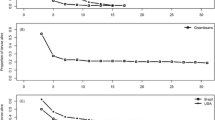
Plant chemical defense and coevolved detoxification mechanisms in specialized herbivorous insects are fundamental in determining many insect–plant interactions. For example, Brassicale plants protect themselves from herbivory by producing glucosinolates, but these secondary metabolites are effectively detoxified by larvae of Pierid butterflies. Nevertheless, not all Brassicales are equally preferred by these specialist herbivores. Female Pieris butterflies avoid laying eggs on anthocyanin-rich red foliage, suggesting red color is a visual cue affecting oviposition behavior. In this study, we reared P. brassicae larvae on green and red cabbage leaves, to determine whether foliage color reliably indicates host plant quality. We did not find a difference in survival rates or maximal larval body mass in the two food treatments. However, larvae feeding on red cabbage leaves exhibited significantly lower growth rates and longer durations of larval development. Interestingly, this longer development was coupled with a higher consumption rate of dry food matter. The lower ratio of body mass gain to food consumption in larvae feeding on red cabbage leaves was coupled with significantly higher (ca. 10 %) larval metabolic rates. This suggests that development on red foliage may incur an increased metabolic load associated with detoxification of secondary plant metabolites. Energy and oxygen allocation to detoxification could come at the expense of growth and thus compromise larval fitness as a result of extended development. From an evolutionary perspective, red foliage color may serve as an honest defensive cue, as it reliably indicates the plant’s low quality as a substrate for larval development.




Similar content being viewed by others
References
Agrawal AA, Kurashige NS (2003) A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J Chem Ecol 29:1403–1415
Archetti M (2000) The origin of autumn colours by coevolution. J Theor Biol 205:625–630
Archetti M, Döring TF, Hagen SB, Hughes NM, Leather SR, Lee DW, Lev-Yadun S, Manetas Y, Ougham HJ, Schaberg PG, Thomas H (2009) Unravelling the evolution of autumn colours: an interdisciplinary approach. Trends Ecol Evol 24:166–173
Chew FS, Renwick JAA (1995) Host-plant choice in Pieris butterflies. In: Carde RT, Bell WJ (eds) Chemical ecology of insects, vol 2. Chapman and Hall, New York, pp 214–238
Cooney LJ, van Klink JW, Hughes NM, Perry NB, Schaefer HM, Menzies IJ, Gould KS (2012) Red leaf margins indicate increased polygodial content and function as visual signals to reduce herbivory in Pseudowintera colorata. New Phytol 194:488–497
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Fineblum WL, Rausher MD (1997) Do floral pigmentation genes also influence resistance to enemies? The W locus in Ipomoea purpurea. Ecology 78:1646–1654
Firake DM, Lytan D, Behere GT, Azad Thakur NS (2012) Host plants alter the reproductive behavior of Pieris brassicae (Lepidoptera: Pieridae) and its solitary larval endo-parasitoid, Hyposoter ebeninus (Hymenoptera: Ichneumonidae) in a cruciferous ecosystem. Fla Entomol 95:905–913
Frazier MF, Wood HA, Harrison JF (2001) Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol Biochem Zool 75:641–650
Fritz VA, Justen VL, Bode AM, Schuster T, Wang M (2010) Glucosinolate enhancement in cabbage induced by jasmonic acid application. HortScience 45:1188–1191
Gerchman Y, Dodek I, Petichov R, Yerushalmi Y, Lerner A, Keasar T (2011) Beyond pollinator attraction: extra-floral displays deter herbivores in a Mediterranean annual plant. Evol Ecol 26:499–512
Gotthard K (2000) Increased cost of predation as a cost of high growth rate: an experimental test in a butterfly. J Anim Ecol 69:896–902
Gould KS, Kuhn DN, Lee DW, Oberbauer SF (1995) Why leaves are sometimes red. Nature 378:241–242
Hamilton WD, Brown SP (2001) Autumn tree colours as a handicap signal. Proc R Soc Lond Ser B: Biol Sci 268:1489–1493
Hughes NM, Smith WK, Gould KS (2010) Red (anthocyanic) leaf margins do not correspond to increased phenolic content in New Zealand Veronica spp. Ann Bot 105:647–654
Irwin RE, Strauss SY, Stortz S, Emerson A, Guibert G (2003) The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology 84:1733–1743
Johnson ET, Berhow MA, Dowd PF (2008) Colored and white sectors from star-patterned petunia flowers display differential resistance to corn earworm and cabbage looper larvae. J Chem Ecol 34:757–765
Jones RE, Hart JR, Bull GD (1982) Temperature, size and egg production in the cabbage butterfly, Pieris rapae L. Aust J Zool 30:223–232
Karageorgou P, Buschmann C, Manetas Y (2008) Red leaf color as a warning signal against insect herbivory: honest or mimetic? Flora 203:648–652
Lee DW, Brammeier S, Smith AP (1987) The selective advantages of anthocyanins in developing leaves of mango and cacao. Biotropica 19:40–49
Lev-Yadun S, Gould KS (2009) Role of anthocyanins in plant defence. In: Gould K, Davies K, Winefield C (eds) Anthocyanins: biosynthesis, functions and applications. Springer, New York, pp 21–48
Lighton JRB (2008) Measuring metabolic rates: a manual for scientists. Oxford University Press, New York
Markwick NP, Poulton J, Espley RV, Rowan DD, McGhie TK, Wadasinghe G, Wohlers M, Jia Y, Allan AC (2013) Red-foliaged apples affect the establishment, growth and development of the light brown apple moth Epiphyas postvittana. Entomol Exp Appl 146:261–275
Mehrkhou F, Mahmoodi L, Mouavi M (2013) Nutritional indices parameters of large white butterfly Pieris brassicae (Lepidoptera: Pieridae) on different cabbage crops. Afr J Agric Res 8:3294–3298
Metspalu L, Hiiesaar K, Joudu J, Kuusik A (2003) Influence of food on the growth, development and hibernation of large white butterfly (Pieris brassicae). Agron Res 1:85–92
Mickel CE (1973) John Ray: indefatigable student of nature. Annu Rev Entomol 18:1–16
Nijhout HF (2003) The control of body size in insects. Dev Biol 261:1–9
Poelman EH, Galiart RJFH, Raaijmakers CE, van Loon JJA, van Dam NM (2008) Performance of specialist and generalist herbivores feeding on cabbage cultivars is not explained by glucosinolate profiles. Entomol Exp Appl 127:218–228
Radcliffe EB, Chapman RK (1966) Varietal resistance to insect attack in various cruciferous crops. J Econ Entomol 59:120–125
Renwick JAA, Chew FS (1994) Oviposition behavior in Lepidoptera. Annu Rev Entomol 39:377–400
Rosen CJ, Fritz VA, Gardner GM, Hecht SS, Carmella SG, Kenney PM (2005) Cabbage yield and glucosinolate concentrations as affected by nitrogen and sulfur fertility. HortScience 40:1493–1498
Schaefer HM, Rolshausen G (2006) Plants on red alert: do insects pay attention? BioEssays 28:65–71
Schoonhoven LM (1990) Host-marking pheromones in Lepidoptera, with special reference to two Pieris spp. J Chem Ecol 16:3043–3052
Schoonhoven LM, Jermy T, van Loon JJA (1998) Insect–plant biology: from physiology to evolution. Chapman and Hall, New York
Smallegange RC, van Loon JJA, Blatt SE, Harvey JA, Agerbirk N, Dicke M (2007) Flower vs. leaf feeding by Pieris brassicae: glucosinolate-rich flower tissues are preferred and sustain higher growth rates. J Chem Ecol 33:1831–1844
Tigreros N (2013) Linking nutrition and sexual selection across life stages in a model butterfly system. Funct Ecol 27:145–154
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Wheat CW, Vogel H, Wittstock U, Braby MF, Underwood D, Mitchell-Olds T (2007) The genetic basis of plant–insect coevolutionary key innovation. Proc Natl Acad Sci USA 104:20427–20431
Whittaker R, Feeny P (1971) Allelochemics: chemical interactions between species. Science 171:757–770
Williams IS (1999) Slow growth- high mortality- a general hypothesis, or is it? Ecol Entomol 24:490–495
Wittstock U, Agerbirk N, Stauber EJ, Olsen CE, Hippler M, Mitchell-Olds T, Gershenzon J, Vogel H (2004) Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc Natl Acad Sci USA 101:4859–4864
Zalucki MP, Clarke AR, Malcolm SM (2002) Ecology and behavior of first instar larval Lepidoptera. Annu Rev Entomol 47:361–393
Acknowledgments
We would like to thank Prof. Simcha Lev-Yadun, Dr. Shu-Ping Huang and two anonymous reviewers for useful comments that helped improve a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Robert Glinwood.
Rights and permissions
About this article
Cite this article
Maskato, Y., Talal, S., Keasar, T. et al. Red foliage color reliably indicates low host quality and increased metabolic load for development of an herbivorous insect. Arthropod-Plant Interactions 8, 285–292 (2014). https://doi.org/10.1007/s11829-014-9307-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-014-9307-2