Abstract
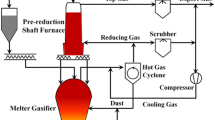
Metallic iron used in steel industries is mostly obtained from a direct reduction process. The focus of this study is to simulate the furnace of the MIDREX technology. MIDREX technology which is the most important gas-based direct reduced iron (DRI) process in the world, includes reduction, transition and cooling zones. The reduction zone considered as a counter current gas–solid reactor produces sponge iron from iron ore pellets. The transition zone has sufficient height to isolate the reduction zone and cooling zone from each other and the cooling zone cools the solid product down to around 50°C. Each zone has a system of reactions. Simultaneous mass and energy balances along the reduction zone lead to a set of ordinary differential equations with two points of boundary conditions. The transitions and cooling zone are investigated at the equilibrium condition leading to a set of algebraic equations. By solving these systems of equations, we determined the materials concentration, temperature, and pressure along the furnace. Our results are in a good agreement with data reported by Parisi and Laborde (2004) for a real MIDREX plant. Using this model, the effect of reactor length and cooling gas flow on the metallization and the effect of cooling gas flow on the outlet temperature of the solid phase have been studied. These new findings can be used to minimize the consumed energy.






Similar content being viewed by others
Abbreviations
- A p :
-
pellet external area (m2)
- C :
-
reactor gas concentration (mol/m3)
- C p :
-
heat capacity (j/mol K)
- D :
-
diffusion constant
- h :
-
global heat transfer coefficient (pellets/gas)
- k :
-
kinetics constant of the surface reaction
- k g :
-
external mass transfer coefficient (m/s)
- L :
-
reduction zone length (m)
- M :
-
metalizing percent
- M w :
-
molecular weight
- n p :
-
number of pellets per unit volume
- P :
-
pressure (bar-g)
- Q m :
-
molar flow (mol/m2s)
- R :
-
reaction rate
- r 0 :
-
external radius of the pellet (m)
- r c :
-
radius of unreacted core (m)
- T :
-
temperature (°C)
- u :
-
velocity (m/s)
- X :
-
extent of reaction/extent of reactant conversation (mol/m3)
- z :
-
space variable inside the reactor (m)
- g :
-
gas
- i, j :
-
counter
- in:
-
reactor inlet
- n :
-
number of carbon atoms
- s1:
-
reactive solid
- s2:
-
product solid
- v :
-
gaseous reactant
- α:
-
stiochiometric coefficient
- εmf :
-
axial voidage
- ρ:
-
density (g/m3)
References
MIDREX Tech. (2009). http://www.midrex.com/.
Hytempsys, Chapter 1 (2009).http://www.energiron.com/Tour/HYLDRMinimillQTVRtour.
D.R. Parisi and M.A. Laborde, Chem. Eng. J. 104, 35 (2004).
A. Shams, A.M. Dehkordi, and I. Goodarznia, Energy Fuels J. 22, 570 (2008).
K.O. Yu and P.P. Gillis, Metall. Trans. B 128, 111 (1981).
W.D. Munro and N.R. Amundson, Ind. Eng. Chem. 42, 1481 (1950).
N.R. Amundson, Ind. Eng. Chem. 48, 26 (1956).
C.W. Siegmund, W.D. Munro, and N.R. Amundson, Ind. Eng. Chem. 48, 43 (1956).
R.J. Schaefer, D. Vortmeyer, and C.C. Watson, Chem. Eng. Sci. 29, 119 (1974).
H. Yoon, J. Wei, and M.M. Denn, AIChE J. 24, 885 (1978).
N.R. Amundson and L.E. Arri, AIChE J. 24, 87 (1978).
B. Alamsari, S. Torii, Y. Bindar, and A. Trianto, Proceeding of International Conference on Computer Engineering and Applications ICCEA in Indonesia (2010), pp. 479–483.
E. Kawasaki, J. Sanscrainte, and T.J. Walsh, AIChE J. 8, 48 (1962).
C.P. La Singh and D.N. Saraf, Ind. Eng. Chem. Process Des. Dev. 18, 364 (1979).
R.H. Perry and D.W. Green, Perry’s Chemical Engineer’s Handbook, 7th ed. (New York, NY: Mc. Graw-Hill, 1999).
R.G. Rice and D.D. Do, Applied Mathematical and Modeling for Chemical Engineers (New York, NY: John Wily & Sons, 1995).
M. Motlagh, Ironmaking Steelmaking 21, 291 (1994).
G.K. Roy and P. Sen Gupta, Ind. Eng. Chem. Proc. Des. Dev. 13, 219 (1974)
G.K. Roy and K.J.R. Sarma, IE (I) J.-CH. 57, 122 (1977).
K. Taylor, A.G. Smith, S. Ross, and M. Smith, Second International Conference on CFD in the Minerals and Process Industries CSIRO, Melbourne, Australia (1999), pp. 273–280.
P. Guimard, D. McNerny, E. Saw, and A. Yang, Pressure Drop for Flow through Packed Beds, (Transport Process Laboratory, Carnegie Mellon University, 2004) pp. 06–363.
D. Acharya, IE (I) J. MC. 85, 219 (2005).
M.A. Rakib and K.I. Alhumaizi, Energy Fuels J. 19, 2129 (2005).
O. Karabelchtchikova (A dissertation submitted to faculty of the Worcester Polytechnic Institute, Worcester, MA, USA, 2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shams, A., Moazeni, F. Modeling and Simulation of the MIDREX Shaft Furnace: Reduction, Transition and Cooling Zones. JOM 67, 2681–2689 (2015). https://doi.org/10.1007/s11837-015-1588-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1588-0