Abstract
Aerosol particulate matter (PM10 and PM2.5) and trace gases (SO2, NO2, CO and O3) were sampled at five locations in greater Dhaka, Bangladesh, between January and April 2006. Particulate matter was collected on micro-fiber filters with a low-volume sampler, and trace gases (SO2, NO2, and O3) were collected with an impinger equipped with PM samplers. Carbon monoxide was determined using the Indicator Tube method. The total average concentrations of SO2, NO2, CO, and O3 were 48.3, 21.0, 166.0 and 28 μg m–3, respectively. The total average concentrations of SO2 and NO2 were much lower than the annual average guideline values of the World Health Organization (WHO). The total average O3 concentration was also much lower than the daily maximum values established by WHO (average of 100 μg m–3 for an 8-h sample). The total average concentrations of five sites were 263, 75.5 and 66.2 μg m–3 for SPM, PM10 and PM2.5, respectively. The mass of PM2.5 is approximately 88% of the PM10 mass, indicating that fossil fuel is the main source of PM in Dhaka. An atomic absorption spectrophotometer was used to determine the heavy metal concentrations in the PM2.5 size fraction. The total average concentrations of As, Cd, Cu, Fe, Pb, and Zn in PM2.5 were 6.3, 13, 94, 433, 204, and 381 ng m–3, respectively. The Pb concentration in Dhaka shows a decreasing tendency, presumably due to the ban on the use of leaded fuel. The overall trace metal concentrations in Dhaka are higher than those in European (e.g., Spain, Norway) and East Asian (e.g., Taiwan) locations, but lower than those measured in Southeast Asian (Kanpur, Delhi, Mumbai, India; Lahore, Pakistan) cities.
Similar content being viewed by others
Introduction
Air pollution has a great impact on human health, climate change, agriculture, and the natural ecosystem (Decker et al. 2000; Mayer et al. 2000; Molina and Molina 2004; Molina et al. 2004). The modernization and industrialization of developing countries has led to the increased use of fossil fuels and their derivatives. As such, developing countries are confronted with the great challenge of controlling the atmospheric pollution, especially in the rapidly growing megacities. Concern about air pollution in urban regions is receiving increasingly importance worldwide, especially pollution by gaseous and particulate trace metals (Azad and Kitada 1998; Salam et al. 2003; Begum et al. 2004; and Cachier et al. 2005).
Gaseous pollutants have major negative impacts on health. They also play an important role in environmental changes and changes in atmospheric chemistry. SO2 and NO2 form acids through different chemical reactions in the atmosphere, and these acids are subsequently deposited on land and ocean surfaces as acid rain. It is anticipated that the increasing load of atmospheric sulfur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO), and ozone (O3) will contribute to global climate change; consequently, it is necessary to quantify the emissions in the very near future.
A great deal of attention has focused on particulate matter (PM) pollution due to their severe health effects, especially fine particles. Several epidemiological studies have indicated a strong association between elevated concentrations of inhalable particles (PM10 and PM2.5) and increased mortality and morbidity (Perez and Reyes 2002; Lin and Lee 2004; Namdeo and Bell 2005). Particulate matter pollution in the atmosphere primarily consists of micron and sub-micron particles from anthropogenic (motor vehicles, biomass, fossil fuel burning) and natural sources (windblown soils and sea spray) (Cohen 1998). The characterization of fine particles has become an important priority of governments, regulators, and researchers due to their potential impact on health, climate, global warming, and long-range transport (Dockery et al. 1993; IPCC 2001).
Dhaka, Bangladesh, is one of the densely populated megacities in the world, with about 15 million inhabitants within a 300-km2 area. Dhaka also suffers from major traffic congestion from both public and private sources. A high concentration of air pollutants, such as black carbon, has already been reported in Dhaka (Salam et al. 2003; Begum et al. 2004). Vehicular emissions, biomass burning for cooking, brick kilns, and construction activities in and around the city are the main contributors to aerosol pollution in Dhaka (Azad and Kitada 1998).
Here, we report the occurrence and characteristics of gaseous (SO2, NO2, CO and O3) pollutants, particulate matter [suspended (S)PM, PM10 and PM2.5), and heavy metal concentrations [arsenic (As), cadmium (Cd), copper (Cu), iron (Fe), lead (Pb), and inc (Zn)] from January to April, 2006 at five locations in the greater Dhaka area.
Sampling and experimental methods
Sampling locations in Dhaka, Bangladesh
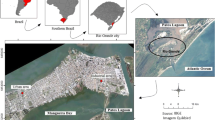
Bangladesh is situated in the eastern part of South Asia. It is surrounded by India on the west, the north, and the northeast, Myanmar on the southeast, and the Bay of Bengal on the south (Fig. 1). Dhaka (23′76°N, 90′38°E, 8 m a.s.l.) is the capital of Bangladesh. It is also the center of commerce and industries of Bangladesh and is growing rapidly—together with all the problems associated with a megacity. Dhaka is situated in flat land surrounded by rivers (Fig. 1). The five sampling locations were selected to reflect the influences from industrial, commercial, residential, and heavy traffic sources in the highly populated greater Dhaka area: Tower Bhaban (Dhaka University campus), Medinova hospital (Dhanmondi), Noverties Pharmaceuticals (Tejgaon), BCIC Bhaban (Motijheel), and Jahangirnagar University campus (Savar). The Jahangirnagar University (JU) campus site is about 30 km northwest of Dhaka city center and considered to be an urban background site. The name of the sampling sites and their characteristics are given in Table 1.
a Map of Bangladesh, b Dhaka city map, c map of Dhaka Division showing the location of the sampling sites. Numbers on black solid square refer the sampling sites: 1 BCIC Bhaban, Motijheel, 2 Tower Bhaban, Dhaka University (DU) campus, Ramna, 3 Medinova hospital, Dhanmondi, 4 Noverties Pharmaceutical, Tejgaon, 5 Jahangirnagar University campus
Meteorology of Dhaka, Bangladesh
The climate of Bangladesh is characterized by high temperatures, excessive humidity, and distinctly marked seasonal variations in precipitation. Bangladesh can be divided into four seasons: pre-monsoon (March–May), monsoon (June–September), post-monsoon (October–November), and winter (December–February). The average meteorological conditions of Dhaka city during the different seasons have been reported by Salam et al. (2003) and Begum et al. (2004). The average winter temperature varies between 9 and 25°C, and the average summer temperature varies from 24 to 34°C. The highest relative humidity occurs in July, reaching 99%, and the lowest occur in December, when it is 36%. Moderately higher temperatures are generally observed during the afternoon. Light precipitation occurs during March and April. The average wind velocity during the sampling period at Dhaka was 1.20 m s‒1 (source: Bangladesh Meteorological Department). Backward air trajectories studies (NOAA/ARL 2005) indicated that air masses were transported from north to southeast (January and February) and also south or southwest to north or northwest (March and April).
Particulate matter and trace gas sampling
Particulate matter and trace gas samplings were performed between January and April 2006 at five locations in Dhaka, Bangladesh (Table 1) using a respirable dust sampler (model 460 NL; Enviro-Tech, Rancho Cordova, CA) for PM10, a fine particulate sampler (model APM 550; Enviro-Tech) for PM2.5, and an organic vapor sampler (model APM 850; Enviro-Tech) for CO measurements with the Indicator Tube method. The PM sampler (model 460 NL; Enviro-Tech) was equipped with impingers to collect the gaseous gases (SO2, NO2, and O3). Ambient air laden with suspended particulates enters the respirable dust sampler through an inlet pipe. As the air enters the cyclone, coarse, non-respirable dust is separated from the air by centrifugal forces acting on the solid particles. These coarse particulates fall through the cyclone and are collected in the sampling bottle fitted at the bottom. The air stream passing through the glass micro-fiber filter (Whatman, Maidstone, UK; size: 20.3 × 24.4 cm,) paper, which was clamped between the top cover and filter adapter assembly, carries the fine dust forming the respirable fraction (PM10). The flow rate of PM collection was determined from the difference of the initial and final flow rate of the instrument gas meter reading. The PM2.5 and PM10 were deposited on micro-fiber filters (Millipore, Bedford, MA), and those larger than PM10 were collected in the dust Cup Vail. Total SPM was calculated from the sum of the PM10 and PM larger than PM10. A regular calibration is necessary to ensure that the sample collection with both instruments is reliable for PM10 (Enviro-Tech model 460 NL) and PM2.5 (EnviroTech model APM 550). A factory calibration was carried out before setting up the instruments for the field measurement. It is absolutely necessary to calibrate the instruments once every 6 months if the instruments are operating 2–3 days per week. The calibrations were done directly by the manufacturer (Envirotech Instruments, New Delhi, India). Sampling instruments were set up on a traffic van (mini truck) for collecting gaseous (SO2, NO2, and O3) and PM from morning to evening (9:00 a.m. to 5:00 p.m. local time), except for CO. The CO samples were collected for 1 h. The sampling instruments were placed about 3 m above ground level. The collected samples were brought to the Inorganic and Analytical Research laboratory, Department of Chemistry, Dhaka University. Trace gas (SO2, NO2, CO, and O3) samples were analyzed immediately. The PM filters were equilibrated for 48 h at a controlled temperature (22 ± 2°C) and relative humidity (55 ± 5%) prior to weighing and then stored in the refrigerator for further trace metal analysis.
Determination of trace gases
SO2 determination
The method for measuring SO2 was adapted from West and Gaeke (1956). In this method, air-exposed samples are treated in a solution of potassium tetrachloro-mercurate, and the concentration of SO2 can be measured in the range of 20–1050 µg m–3. A dichlorosulphitomercurate complex is formed, which subsequently reacts with pararosaniline and formaldehyde to form the intensely colored pararosaniline methylsulphonic acid (Lyles et al. 1965). We measured the absorbance of the colored solution with an UV-spectrophotometer (model AA-680; Shimazdu, Japan) at 560 nm, and the concentration of SO2 was determined based on a calibration curve of five standard solutions ( 0.0, 4.155, 8.29, 16.0, 24.87, and 33.16 µg SO2/25 ml). A blank sample was prepared using the same procedure as that for the air-exposed sample for the blank correction.
NO2 determination
The principle of NO2 measurement in atmospheric samples was described by Jacob and Hochheiser (1958); the normal range of this method is 9–750 μg m–3. In this method, air-exposed samples were treated with sodium hydrogen and sodium arsenite. The sample solution was again treated with phosphoric acids, sulfanilamide, and a N-(1-naphthyl)-ethylenediamine di-hydrochloride (NEDA) solution. A colored solution was produced, and the absorbance of the colored solution was measured with a UV-spectrophotometer (model AA-680l Shimazdu) at 540 nm. The concentrations of NO2 were determined from the calibration curve of five standard solutions (0.0, 1.0, 2.0, 3.0, 5.0, 7.0, and 9.0 µg NO2/50 ml). A blank sample was also prepared using the same procedure as that for the air-exposed sample for the blank correction.
CO determination
The concentration of CO was determined by Indicator Tube Method. The air was passed through the tube by an aspirator. After 1 h of sampling, the color of the fixed portion of the tube was matched with given chart value (APM 850 model; Enviro-Tech, India). The concentration was calculated using the equations: \({\text{CO concentration }}{\left( {\mu {\text{gm}}^{{ - {\text{3}}}} } \right)} = {\left[ {{{\left( {{\text{Chart value of 1 Squeeze}} \times {\text{0}}{\text{.75}} \times {\text{10}}^{{\text{6}}} } \right)}} \mathord{\left/ {\vphantom {{{\left( {{\text{Chart value of 1 Squeeze}} \times {\text{0}}{\text{.75}} \times {\text{10}}^{{\text{6}}} } \right)}} {\text{F}}}} \right. \kern-\nulldelimiterspace} {\text{F}}} \right]}\), where 0.75 is the factor for the density of CO, 106 is the conversation factor for both air volume (from liter to cubic meter) and milligram to microgram, and F is the total volume for air passed (l) through the indicator tube. The total volume of air passed through the CO indicator tube = average flow rate (lpm) x time (min).
O3 determination
An absorbing reagent was prepared with KH2PO4, Na2HPO4, and KI for O3 concentration determination. The air-exposed samples were treated with the absorbing reagents, which produced a colored solution. The absorbance of the colored solutions was measured at 352 nm against a blank using a UV-spectrophotometer (model AA-680; Shimazdu). The concentration of O3 was measured from a calibration curve of five standard solutions (0.0, 1.0, 3.0, 5.0, 7.0, and 9.0 µg/25 ml). Blank correction was performed by preparing a blank sample using the same procedure as that used for the air-exposed samples.
Determination of PM
The aerosol masses for PM2.5 and PM10 were determined by weighing the filters before and after exposure at ambient temperature and atmospheric pressure with an OHAUS analytical balance (model no. AR1140; OHAUS, Pine Brook, NJ). Total suspended particulate (TSP) matter was determined from the sum of PM10 and particles larger than PM10. The mass of PM larger than PM10 was determined from the initial and final weight of the dust Cup Vail. Samples were transported from the sampling site to the laboratory and kept them in a refrigerator until analysis to minimize volatilization. Blank correction was performed by preparing field blanks using the same procedure as that for the PM samples.
Determination of heavy metals
An atomic absorption spectrophotometer (AAS; model AA-680; Shimadzu) was used to determine the concentrations of heavy metals (As, Cd, Cu, Fe, Pb, Zn) in the PM2.5 size fraction during the period January–April 2006. The PM2.5-loaded filters were extracted in an acid mixture to remove heavy metals. Each filter (diameter 4.7 cm) was placed in 30 ml concentrated nitric acid (70%) and 10 ml hydrogen peroxide (30%) solution in a digestion vessel. The digestion vessels were placed on a sand bath and heated to 180°C for about 1 h until the acid solution had evaporated. The procedure was repeated twice and was continued until the residue was almost dry. Upon cooling, 60 ml water was added and agitated carefully. The solution was filtered into a 100-ml volumetric flask, diluted to the mark with de-ionized water, and used for the trace metal analysis with AAS. One unexposed filter was prepared as a blank using the same procedure as that followed for the air-exposed filter.
For the determination of the heavy metal concentrations, calibration curves for each metal were prepared with five standard solutions at different concentrations. Metal solutions were aspirated into the flame of the AAS, where ions are reduced to the atomic state, and absorbed light can be measured quantitatively at a particular wavelength. However, the concentrations of each metal were determined from the standard calibration curve of the individual metal.
Results and discussion
Overview of the experimental results
Assessment of the concentrations of ambient air PM10 and PM2.5, trace gases (SO2, NO2, CO and O3), and heavy metals (As, Cd, Cu, Fe, Pb, Zn) were determined on PM2.5 size fractions at five locations in Dhaka, Bangladesh from January to April 2006. The sampling sites were located at Tower Bhaban (Dhaka University campus), Medinova hospital (Dhanmondi), Noverties Pharmaceuticals (Tejgaon), BCIC Bhaban (Motijheel), and Jahangirnagar University campus (Savar). The description of the sampling sites are given in Table 1 and also shown in Fig. 1. The concentrations of measured components varied between residential, industrial, and semi-urban sites in the greater Dhaka area and also varied slightly temporally between January and April 2006. The concentrations of trace gases remained almost constant over the entire sampling period, whereas those of the PM varied from 1 month to the other, with higher concentrations of both PM10 and PM2.5 observed in January and lower concentrations in April. Details of the experimental results were given in Tables 2, 3, 4.
Trace gases
The atmospheric trace gases (SO2, NO2, CO and O3) were measured at five locations in Dhaka during the period of January to April, 2006. Samples were collected once in a week at each location. The sampling time was 8 h for SO2, NO2, and O3 and 1 h for CO. The samples were collected in an impinger attached to the PM samplers for SO2, NO2, and O3, and an organic vapor sampler was used for CO measurements with the Indicator Tube method. The overall average value of SO2 at five sampling locations in Dhaka was 48 μg m–3, which is very close to the annual average of the World Health Organization’s (WHO) guideline values for the European Union (WHO 2000: 50 μg m–3). Because the total sampling period in our study was about 4 months [8 h daily for all gases except CO (1 h)] and extended from the winter through the beginning of the monsoon season, it can be considered to be an average for the whole year. The highest concentration (76.8 μg m–3) of SO2 was found in the commercial and heavy traffic areas (BCIC Bhaban, Motijheel), and the lowest concentration (20.7 μg m–3) was found in the semi-urban area (Jahangirnagar University campus, Savar). The elevated concentration of SO2 in the city center is probably due to the high content of sulfur in fossil fuel. Azad and Kitada (1998) reported that the primary source of SO2 in Dhaka is traffic fuels (55.8%) followed by brick fields (28.8%), industry (10.5%), and others (about 5%); they also reported high SO2 concentrations in the southern industrial area, where the highest value was about 286 μg m–3.
The total average concentration of NO2 at five locations in Dhaka was 21.0 μg m–3, which is about half of the annual WHO guideline value 2005 (40 μg m–3). The NO2 enters the atmosphere from various natural and anthropogenic sources, including lighting, action of microorganisms on nitrogen-based fertilizer, but the most important and major anthropogenic source is the combustion of fossil fuel in traffic. The highest concentration of NO2 (40 μg m–3) was found at Medinava hospital, Dhanmondi site, with medium traffic, whereas the lowest concentration (5.0 μg m–3) was found at Jahangirnagar University, Savar, a semi-urban location. Azad and Kitada 1998 measured NO2 concentrations with molecular diffusion tubes in Dhaka, Bangladesh. They reported that the highest 10-day average was about 65.8 μg m–3, with higher concentrations appearing in the city center along the main roads of Dhaka. Motor vehicles and brick fields were speculated to be the two major emission sources in Dhaka.
The measured CO values varied between industrial, urban, and semi-urban sites. The highest concentration (334 μg m–3) of CO was observed at the industrial site (Novarties, Tejgaon), whereas the lowest value (42 μg m–3) was found in the semi-urban area (Jahangirnagar University, Savar). The highest concentration of CO at the industrial sites is presumably due to the incomplete conversion of fossil fuel during different mechanical and industrial processes. However, the overall (4 months at five sampling locations) average value of CO in Dhaka was 166 μg m–3 (1-h sampling time daily).
The total average concentration of O3 at five sampling sites in Dhaka was 28 µg m–3, which is much lower than the daily maximum value (100 µg m–3; WHO 2005). However, O3 is one of the most ubiquitous and toxic pollutants found in ambient air. A higher concentration (76.5 µg m–3) of O3 was found in the semi-urban area (Jahangirnagar University campus, Savar), and the lowest concentration (3.92 µg m–3) was found in the industrial area (Noverties, Tejgaon).
Particulate matter
Atmospheric particulate matter (PM10, PM2.5) was collected on micro-fiber filters with low-volume samplers from 9:00 a.m. to 5:00 p.m. (8 h on average) between January and April 2006 at five locations in Dhaka, Bangladesh. Total suspended particulate matter was calculated from the sum of the PM10 and particles larger than PM10 (collected in the dust Cup Vail). The total average concentration of SPM in the ambient air of Dhaka was 262.6 μg m–3. The World Bank and Department of the Environment of Bangladesh found SMP concentrations of 665–2456 μg m–3 at Farmgate, Dhaka (Core 1998) based on 8-h measurements at several locations along busy roads in Dhaka city. The difference between these 1998 results and those of our study indicates that the ambient concentrations of SPM in Dhaka are decreasing remarkably. However, our measurements showed that the maximum daily average (exposure time: 8 h daily) concentration of SPM was 570 μg m–3 at Motijheel in January, 2006. The SPM concentrations among the five sampling sites varied from 126.2 μg m–3 at Jahangirnagar University (JU) campus, Savar to 523.0 μg m–3 at BCIC Bhaban, Motijheel (Table 3). The causes of the higher SPM values in Dhaka may be road dusts, construction activities, incomplete combustion of fossil fuel from traffic vehicles and brick kilns, and long-range transport (Begum et al. 2004).
The total average concentrations of PM10 and PM2.5 were 75.5 and 66.2 μg m–3, respectively, at five locations in the greater Dhaka area. At the five sampling locations in Dhaka, the average mass concentrations varied from 62.0 to 91.2 μg m–3 for PM10, whereas these ranged between 54.2 and 80.5 μg m–3 for PM2.5 (Table 3). Chuersuwan et al. (2008) reported mass concentrations of 57.6–108.1 μg m–3 for PM10 and between 69.0 and 37.9 μg m–3 for PM2.5 at four sampling sites in Bangkok, Thailand. The PM10 mass concentrations in Kanpur, India were much higher than those we found for Dhaka. At three sampling locations in Kanpur, the average PM10 mass concentration was 211 μg m–3 and ranged from 80 to 281 μg m–3 (Sharma and Maloo 2005). The average PM2.5 mass concentration at the same locations, 101 μg m–3, was also much higher than that at Dhaka, ranging from 65 to 146 μg m–3 (Sharma and Maloo 2005). The ratio between PM2.5 and PM10 in Dhaka shows that particulate mass mostly derived from fine fraction, which is very harmful for human health. PM2.5 mass is about 88% of that of PM10, which indicates that the source of PM is mostly from fossil fuel. Begum et al. 2004 also reported that vehicles normally produce more fine particles (PM2.5) than coarse ones (PM2.5–10), with the latter mostly originating from mechanical processes. The concentrations of the coarse particles (PM2.5–10) varied from 12.0 to 9.31 μg m–3, and the average concentration for the coarse fraction was 9.31 μg m–3 (Table 3). However, the elevated concentrations of PM observed in Dhaka, Bangladesh are still lower than those reported for Southeast Asian sites (Smith et al. 1996; Sharma and Maloo 2005).
Heavy metals
The concentrations of selected trace metals were determined at five locations in the greater Dhaka region for PM2.5 in the period from January to April 2006. PM2.5 samples were collected on fiber filters (diameter 4.7 cm) with a low-volume sampler. An AAS was used to determine the concentrations of heavy metals (As, Cd, Cu, Fe, Pb, Zn). The measured concentrations of the heavy metals are given in Table 4.
Arsenic
Arsenic is a major ground water pollutant in many parts of Bangladesh. Millions of people are affected by As-related diseases in Bangladesh due to contaminated drinking water. It is therefore very important to check the As concentrations in the air of Bangladesh. The total average As concentration at the five locations in Dhaka was 6.3 ng m–3. However, the concentration of As varied from 4.3 ng m–3 (Medinova hospital, Dhanmondi) to 7.9 ng m–3 (Noverties Pharmaceutical, Tejgaon) (Table 4). Unexpectedly high As concentrations (325 ng m–3) were observed at all four sites in Bangkok, Thailand (Chuersuwan et al. 2008).
Cadmium
Average Cd concentrations ranged from 0.2 to 34.9 ng m–3. The lowest value among these five sites was found at Tower Bhaban, Dhaka University campus (residential area), whereas the highest value was observed at Noverties Pharmaceutical, Tejgaon Industrial area. The higher Cd concentration at the industrial site may be due to the release of Cd from different industrial mechanical processes. The total average of Cd in Dhaka (13 ng m–3) is slightly higher than that previously reported by Salam et al. 2003, is comparable with those found in Spain (Querol et al. 2002), but if three- to fourfold lower than the values reported for other Southeast Asian sites (Sharma and Patil 1992; Smith et al. 1996).
Copper
The average Cu concentrations varied from 31 ng m–3 (Tower Bhaban, Dhaka University campus) to 132 ng m–3 (Medinova hospital, Dhanmondi) (Table 4). The average value of Cu was 95 ng m–3 for PM2.5, which is about twofold higher than the value (54 ng m–3 for TSP) obtained by Salam et al. 2003. The average Cu concentration of five locations in Dhaka, Bangladesh for PM2.5 was slightly higher than that (65 ng m–3) found at four sites in Bangkok, Thailand (Chuersuwan et al. 2008).
Iron
The average Fe concentration varied from 225 ng gm–3 (Jahangirnagar University, Savar) to 669 ng m–3 (Noverties Pharmaceutical, Tejgaon). The highest value of Fe (669 ng m–3) was observed at the Tejgaon industrial area, probably due to the industrial activities there. The lower value of Fe (225 ng m–3) at Jahangirnagar University Campus may be due to the lack of industrial activity at this site and also because there is less traffic than at the Noverties Pharmaceutical, Tejgaon site. Iron exhibited relatively higher values at all five sites. The average estimated value of Fe was 433 ng m–3. While Fe may originate from soil dusts, poorly managed transport, and building construction, among others, the major sources of Fe are both anthropogenic and crustal in origin, including iron and steel manufacturing units and the weathering of exposed Fe in urban areas. However, the overwhelming source of Fe particles in the atmosphere is from crustal weathering. The average value of Fe was 433 ng m–3, which is higher than the concentration found in Los Angeles, USA and Spain (Querol et al. 2002) and also lower than that reported for Asian sites (Fung and Wong 1995; Balachandran et al. 2000).
Lead
The average Pb concentration varied from 51 ng m–3 (Jahangirnagar University, Savar) to 335 ng m–3 (BCIC Bhaban, Motijheel) (Table 4). The higher value of Pb may be due to the presence of heavy traffic at the Motijheel area, while the lower value may be due to relatively lower level of traffic. Lead compounds accumulate with PM in the atmosphere and gradually settle on the Earth’s surface. The level of Pb in rural areas in Bangladesh was found to be below the detection limit (Salam et al. 2003). We found the total average concentration of Pb at the five sites in Dhaka to be 204 ng m–3 of PM2.5 (Table 4), which is about half the WHO 1999 guideline value of 500 ng m–3 for urban areas. Scientists at the Bangladesh Atomic Energy Commission (BAEC) observed that Dhaka was the most Pb-polluted city in the world for a part of 1996. A 17-month survey conducted by the scientists of BAEC detected 463 ng m–3 Pb for PM2.5 in Dhaka air during the dry months (between November 1994 and January 1996) (Khaliquzzaman et al. 1997). However, the atmospheric Pb concentration in Dhaka is decreasing gradually, presumably due to the ban on leaded gasoline in Bangladesh, although it is still higher than that found in European cities (NILU 2002; Manalis et al. 2005) and Far East Asian sites Taiwan (133 ng m–3 by Fung and Wong 1995). However, it is lower than that reported for Southeast Asian Sites such as Karachi, Pakistan, and Delhi, Mumbai, and Kanpur, India (Smith et al. 1996; Balachandran et al. 2000; Kumar et al. 2001; Sharma and Maloo 2005).
Zinc
The average Zn concentrations varied from 103 ng m–3 (Jahangirnagar University, Savar) to 516 ng m–3 (Noverties Pharmaceuticals, Tejgaon) (Table 4). The highest value of Zn at Noverties Pharmaceuticals was probably due to the industrial release of Zn at this location, while the lowest value at Jahangirnagar University campus may be the result of relatively less traffic. The average value of Zn for PM2.5 was 381 ng m–3, which is much lower than the value (801 ng m–3) obtained at a previous measurement by Salam et al. 2003 for TSP. Elevated concentrations of Zn have also been observed for other Southeast Asian sites (e.g., Lahore, Pakistan; Smith et al. 1996). The Zn concentration at Dhaka was much lower than that at Delhi (Balachadran et al. 2000) and Kanpur (Sharma and Maloo 2005), India, but much higher than that found in Norway (NILU 2002).
Conclusions
Atmospheric pollution at five locations in greater Dhaka, Bangladesh, was characterized in terms of trace gases, PM, and heavy metals. The average concentration of O3 in the greater Dhaka area was lower than the WHO guidelines value (WHO 2005). Elevated concentrations of PM were observed in Dhaka, but these were still lower than those reported for Southeast Asian sites. Particulate mass originates mostly from fine particles (about 88% of PM10 mass was from PM2.5). The concentrations of Pb, Zn, Cu, Fe, As, and Cd were much lower than the previous measurements in Dhaka city. In particular, the Pb concentration is decreasing due to ban of leaded fuel in Bangladesh.
References
Azad AK, Kitada T (1998) Characteristics of the air pollution in the city of Dhaka, Bangladesh in winter. Atmos Environ 32:1991–2005. doi:10.1016/S1352–2310(97)00508–6
Balachandran S, Meena BR, Khillare PS (2000) Particle size distribution and its elemental composition in the ambient air of Delhi. Environ Int 26:49–54. doi:10.1016/S0160–4120(00)00077–5
Begum BA, Kim E, Biswas SK, Hopke PK (2004) Investigation of sources of atmospheric aerosol at urban and semi-urban areas in Bangladesh. Atmos Environ 38:3025–3038. doi:10.1016/j.atmosenv.2004.02.042
Cachier H, Aulagnier F, Sarda R, Gautier F, Masclet P, Besombes JL et al (2005) Aerosol studies during the ESCOMPTE experiment: an overview. Atmos Res 74:547. doi:10.1016/j.atmosres.2004.06.013
Chuersuwan N, Nimrat S, Lekphet S, Kerdkumrai T (2008) Levels and major sources of PM2.5 and PM10 in Bangkok Metropolitan Region. Environ Int 34:671–677. doi:10.1016/j.envint.2007.12.018
Cohen DD (1998) Characterization of atmospheric fine particle using IBA techniques. Nucl Instrum Methods 136:14–22. doi:10.1016/S0168–583X(97)00658–7
Core J (1998) Sources of air pollution and control options. Paper presented in the Consultative Meeting of World Bank and DoE. Government of Bangladesh, Dhaka
Decker EH, Elliot S, Smith FA, Blake DR, Rowland FS (2000) Energy and material flow through the urban environment. Annu Rev Energy Environ 25:685–740. doi:10.1146/annurev.energy.25.1.685
Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME et al (1993) An association between air pollution and mortality in six US citites. N Engl J Med 329:1753–1759. doi:10.1056/NEJM199312093292401
Fung YS, Wong LWY (1995) Apportionment of air pollution sources by receptor models in Hong Kong. Atmos Environ 29:2041–2048. doi:10.1016/1352–2310(94)00239-H
IPCC (International Panel on Climate Change) (2001) The third assessment report of Working Group I of the Intergovernmental Panel on Climate Change. Technical summary. IPCC, Shanghai
Jacob MB, Hochheiser S (1958) Continuous sampling and ultra-micro determination of nitrogen dioxide in air. Anal Chem 30:426–431. doi:10.1021/ac60135a032
Khaliquzzaman M, Biswas SK, Tarafdar SA, Islam A, Khan AH (1997) Trace element composition of size fractionated airborne particulate matter in urban and rural areas in Bangladesh. Report AECD/AFD-CH/6–4
Kumar AV, Patil RS, Nambi KSV (2001) Source apportionment of suspended particulate matter at two traffic junctions in Mumbai, India. Atmos Environ 35:4245–4251. doi:10.1016/S1352–2310(01)00258–8
Lin J, Lee LC (2004) Characterization of the concentration and distribution of urban submicron (PM1) aerosol particles. Atmos Environ 38:469–475. doi:10.1016/j.atmosenv.2003.09.056
Lyles GR, Dowling FB, Blanchard VJ (1965) Quantitative determination of formaldehyde in parts per hundred million concentration level. J Air Pollut Control Assoc 15:106–114
Manalis N, Grivas G, Protonotarios V, Moutsatsou A, Samara C, Chaloulakou A (2005) Toxic metal content of particulate matter (PM10), within the Greater Area of Athens. Chemosphere 60:557–566. doi:10.1016/j.chemosphere.2005.01.003
Mayer M, Wang C, Webster M, Prinn RG (2000) Linking local air pollution to global chemistry and climate. J Geophys Res 105:22 869–22 896
Molina MJ, Molina LT (2004) Megacities and atmospheric pollution. J Air Waste Manage Assoc 54:644–680
Molina LT, Molina MJ, Slott R, Kolb CE, Gbor PK, Meng F et al (2004) Air quality in selected megacities. Crit Rev 10[Suppl]. Available at: http://www.awma.org
Namdeo A, Bell MC (2005) Characteristics and health implications of fine and coarse particulates at roadside, urban background and rural sites in UK. Environ Int 31:565–573. doi:10.1016/j.envint.2004.09.026
NILU (Norwegian Institute of Air Research) (2002) Heavy metals and POPS within the EMEP region 2000. Report -EMEP/CCC-9/2002, Norwegian Institute of Air Research, Kjeller
NOAA/ARL (National Oceanic and Atmospheric Administration/Air Resources Laboratory) (2005) HYSPLIT4 model. Available at: http://www.arl.noaa.gov/ready/hysplit4.html). NOAA Air Resources Laboratory, Silver Spring
Perez P, Reyes J (2002) Prediction of maximum of 24-h average of PM10 concentrations 30 h in advance in Santiago, Chile. Atmos Environ 36:4555–4561. doi:10.1016/S1352–2310(02)00419–3
Querol X, Lastuey A, De La Rosa J, Sanchez A, Plana F, Ruiz CR (2002) Source apportionment analysis of atmospheric particulates in an industrialized urban site in southwestern Spain. Atmos Environ 36:3113–3125. doi:10.1016/S1352–2310(02)00257–1
Salam A, Bauer H, Kassin K, Ullah SM, Puxbaum H (2003) Aerosol chemical characteristics of a mega-city in Southeast Asia (Dhaka, Bangladesh). Atmos Environ 37:2517–2528. doi:10.1016/S1352–2310(03)00135–3
Sharma M, Maloo S (2005) Assessment of ambient air PM10 and PM2.5 and characterization of PM10 in the city of Kanpur, India. Atmos Environ 39:6015–6026. doi:10.1016/j.atmosenv.2005.04.041
Sharma VK, Patil RS (1992) Chemical composition and source identification of Bombay aerosol. Environ Technol 13:1043–1052
Smith DJT, Harrision RM, Luhana L, Casimiro AP, Castro LM, Tariq MN et al (1996) Concentrations of particulate airborne polycyclic aromatic hydrocarbons and metals collected in Lahore, Pakistan. Atmos Environ 30:4031–4040. doi:10.1016/1352–2310(96)00107–0
West PW, Gaeke GC (1956) Fixation of sulphur dioxide as sulfitomercurate III and subsequent colorimetric determination. Anal Chem 28:1816–1819. doi:10.1021/ac60120a005
World Health Organization (WHO) (1999) WHO air quality guidelines for Europe. WHO regional office for Europe, WHO Regional Publications, European Series, Copenhagen
World Health Organization (WHO) (2000) WHO air quality guidelines for Europe 2000, 2nd edn. WHO regional office for Europe, WHO Regional Publications, European Series, Copenhagen
World Health Organization (WHO) (2005) WHO air quality guideline update. Report of the working group meeting. WHO, Bonn, Germany
Acknowledgments
The authors thank to the Ministry of Science Information and Communication Technology for granting a special allocation to carry out the current research work. We gratefully acknowledge two reviewers for their helpful suggestions.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Salam, A., Hossain, T., Siddique, M.N.A. et al. Characteristics of atmospheric trace gases, particulate matter, and heavy metal pollution in Dhaka, Bangladesh. Air Qual Atmos Health 1, 101–109 (2008). https://doi.org/10.1007/s11869-008-0017-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-008-0017-8