Abstract
Purpose of Review
Essential thrombocythemia (ET) and polycythemia vera (PV) are the most common myeloproliferative neoplasms (MPNs). Treatment of ET and PV is based on the risk for subsequent thrombosis. High-risk patients, defined as older than 60, JAK2 V617F–positive patients, or patients with a history of prior thrombosis, merit cytoreduction to control blood counts, whereas a watchful waiting paradigm is utilized in low-risk patients. However, low-risk patients have a host of other specific management issues that arise during their disease course. This review will discuss the most common management issues specific to the care of low-risk patients, including anti-platelet therapy dosing, pregnancy, and indications for early cytoreduction.
Recent Findings
Although low-dose aspirin is well established in PV, its indications and dosing regimens are less clear in ET. Recent evidence has supported twice daily low-dose aspirin in ET and observation alone in very low–risk ET patients. Pregnancy is not contraindicated in MPNs, and we recommend aspirin throughout pregnancy with consideration for prophylactic postpartum anticoagulation. High phlebotomy needs, symptom burden, and extreme thrombocytosis are common reasons for initiation of cytoreduction in low-risk patients, although we typically do not start cytoreduction for an isolated high platelet count alone. Recent data has also demonstrated a potential disease-modifying effect of interferons in MPNs, with some experts now advocating the early use of interferon in low-risk patients, although more mature data is needed before practice guidelines change.
Summary
We evaluate the literature to inform clinical decision-making regarding these controversies, including most recent data that has challenged the “watchful waiting” paradigm. Our discussion provides guidance on common clinical scenarios seen in low-risk ET and PV patients, who face a myriad of complex management decisions in their care.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost. 2007;33(4):313–20.
Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015;5:e369.
Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–32.
Network NCC. Myeloproliferative neoplasms (version 2.2019). https://www.nccn.org/professionals/physician_gls/pdf/mpn_blocks.pdf. Accessed August 17, 2019.
Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332(17):1132–7.
Kaplan ME, Mack K, Goldberg JD, Donovan PB, Berk PD, Wasserman LR. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol. 1986;23(3):167–71.
Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114–24.
Chu DK, Hillis CM, Leong DP, Anand SS, Siegal DM. Benefits and risks of antithrombotic therapy in essential thrombocythemia: a systematic review. Ann Intern Med. 2017;167(3):170–80.
Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood. 2010;116(8):1205–10 (quiz 387).
Alvarez-Larran A, Pereira A, Guglielmelli P, et al. Antiplatelet therapy versus observation in low-risk essential thrombocythemia with a CALR mutation. Haematologica. 2016;101(8):926–31.
Dragani A, Pascale S, Recchiuti A, et al. The contribution of cyclooxygenase-1 and -2 to persistent thromboxane biosynthesis in aspirin-treated essential thrombocythemia: implications for antiplatelet therapy. Blood. 2010;115(5):1054–61.
Rocca B, Ciabattoni G, Tartaglione R, et al. Increased thromboxane biosynthesis in essential thrombocythemia. Thromb Haemost. 1995;74(5):1225–30.
Viallard JF, Solanilla A, Gauthier B, et al. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood. 2002;99(7):2612–4.
Pascale S, Petrucci G, Dragani A, et al. Aspirin-insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. Blood. 2012;119(15):3595–603.
De Stefano V, Rocca B, Tosetto A, et al. The Aspirin Regimens in Essential Thrombocythemia (ARES) phase II randomized trial design: implementation of the serum thromboxane B2 assay as an evaluation tool of different aspirin dosing regimens in the clinical setting. Blood Cancer J. 2018;8(6):49.
Rocca B, Tosetto A, Betti S, et al. A randomized double-blind trial of 3 aspirin regimens to optimize antiplatelet therapy in essential thrombocythemia. Blood. 2020;136(2):171–82. The randomized ARES trial evaluates three dosing regimens of aspirin in ET patients, and found that twice daily and three-times daily aspirin results in greater platelet inhibition of COX1 compared to once daily dosing. Twice daily regimens are better tolerated than three-times daily, with no differences in pharmacologic inhibition. This trial provides early evidence for alternative dosing mechanisms of aspirin in ET, which is an essential component of ET management.
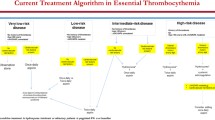
Tefferi A, Vannucchi AM, Barbui T. Essential thrombocythemia treatment algorithm 2018. Blood Cancer J. 2018;8(1):2.
Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–63.
Pourcelot E, Trocme C, Mondet J, Bailly S, Toussaint B, Mossuz P. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: clinical implications. Exp Hematol. 2014;42(5):360–8.
Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–11.
Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33(9):1199–203.
Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–8.
Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–103.
Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778–81.
Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC Cancer. 2016;16:167.
Geyer HL, Scherber RM, Dueck AC, et al. Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: retrospective assessment in 1470 patients. Blood. 2014;123(24):3803–10. This study provides granular analysis of symptom burden profilers in MPN patients. Symptoms can be clustered into distinct groups, which do not not necessarily correspond to risk stratification. Distinct symptom clusters indicate possible biologic subgroups within MPNs, and underscore the importance of routine symptom monitoring in the care of MPN patients.
Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–35.
Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807.
Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood. 2017;130(17):1889–97.
Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33.
De Domenico I, Lo E, Ward DM, Kaplan J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci U S A. 2009;106(10):3800–5.
Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618–28.
Scherber RM, Geyer HL, Dueck AC, et al. The potential role of hematocrit control on symptom burden among polycythemia vera patients: insights from the CYTO-PV and MPN-SAF patient cohorts. Leuk Lymphoma. 2017;58(6):1481–7.
Silver RT, Gjoni S. The hematocrit value in polycythemia vera: caveat utilitor. Leuk Lymphoma. 2015;56(5):1540–1.
Verstovsek S, Harrison CN, Kiladjian JJ, et al. Markers of iron deficiency in patients with polycythemia vera receiving ruxolitinib or best available therapy. Leuk Res. 2017;56:52–9.
Ginzburg YZ, Feola M, Zimran E, Varkonyi J, Ganz T, Hoffman R. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia. 2018;32(10):2105–16.
Kremyanskaya M, Ginzburg Y, Kuykendall AT, et al. PTG-300 Eliminates the need for therapeutic phlebotomy in both low and high-risk polycythemia vera patients. Blood. 2020;136(Supplement 1):33–5.
Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001–12. Br J Haematol. 2016;174(3):382–96.
Alimam S, Bewley S, Chappell LC, et al. Pregnancy outcomes in myeloproliferative neoplasms: UK prospective cohort study. Br J Haematol. 2016;175(1):31–6.
• Maze D, Kazi S, Gupta V, et al. Association of treatments for myeloproliferative neoplasms during pregnancy with birth rates and maternal outcomes: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(10):e1912666. This systematic review and meta-analysis synthesizes recent findings in the literature on the outcomes of pregnant women with MPNs. Pregnancy outcomes are overall excellent with high birth rates. Aspirin and interferon use are associated with improved live birth rates.
Griesshammer M, Sadjadian P, Wille K. Contemporary management of patients with BCR-ABL1-negative myeloproliferative neoplasms during pregnancy. Expert Rev Hematol. 2018;11(9):697–706.
How J, Leiva O, Bogue T, et al. Pregnancy outcomes, risk factors, and cell count trends in pregnant women with essential thrombocythemia. Leuk Res. 2020;98:106459.
Passamonti F, Randi ML, Rumi E, et al. Increased risk of pregnancy complications in patients with essential thrombocythemia carrying the JAK2 (617V>F) mutation. Blood. 2007;110(2):485–9.
Rumi E, Bertozzi I, Casetti IC, et al. Impact of mutational status on pregnancy outcome in patients with essential thrombocytemia. Haematologica. 2015;100(11):e443–5.
Melillo L, Tieghi A, Candoni A, et al. Outcome of 122 pregnancies in essential thrombocythemia patients: a report from the Italian registry. Am J Hematol. 2009;84(10):636–40.
Gangat N, Wolanskyj AP, Schwager S, Tefferi A. Predictors of pregnancy outcome in essential thrombocythemia: a single institution study of 63 pregnancies. Eur J Haematol. 2009;82(5):350–3.
Skeith L, Carrier M, Robinson SE, Alimam S, Rodger MA. Risk of venous thromboembolism in pregnant women with essential thrombocythemia: a systematic review and meta-analysis. Blood. 2017;129(8):934–9.
Beauverd Y, Radia D, Cargo C, et al. Pegylated interferon alpha-2a for essential thrombocythemia during pregnancy: outcome and safety. A case series. Haematologica. 2016;101(5):e182–4.
How C-J, Leiva O, Bogue T, et al. Pregnancy outcomes, risk factors, and gestational cell count trends in pregnant women with essential thrombocythemia and polycythemia vera. Blood. 2019;134(Supplement_1):4172.
Sanchez-Aguilera A, Arranz L, Martin-Perez D, et al. Estrogen signaling selectively induces apoptosis of hematopoietic progenitors and myeloid neoplasms without harming steady-state hematopoiesis. Cell Stem Cell. 2014;15(6):791–804.
Turhan AG, Humphries RK, Cashman JD, Cuthbert DA, Eaves CJ, Eaves AC. Transient suppression of clonal hemopoiesis associated with pregnancy in a patient with a myeloproliferative disorder. J Clin Invest. 1988;81(6):1999–2003.
Harrison C, Baxter J, Boucher RH, et al. Effects of tamoxifen on the mutant allele burden and disease course in patients with myeloproliferative neoplasms - results of the TAMARIN study. Blood. 2020;136(Supplement 1):33–5.
Szuber N, Vallapureddy RR, Penna D, et al. Myeloproliferative neoplasms in the young: Mayo Clinic experience with 361 patients age 40 years or younger. Am J Hematol. 2018;93(12):1474–84.
Sanchez-Luceros A, Meschengieser SS, Woods AI, et al. Acquired von Willebrand factor abnormalities in myeloproliferative disorders and other hematologic diseases: a retrospective analysis by a single institution. Haematologica. 2002;87(3):264–70.
Lancellotti S, Dragani A, Ranalli P, et al. Qualitative and quantitative modifications of von Willebrand factor in patients with essential thrombocythemia and controlled platelet count. J Thromb Haemost. 2015;13(7):1226–37.
Rottenstreich A, Kleinstern G, Krichevsky S, Varon D, Lavie D, Kalish Y. Factors related to the development of acquired von Willebrand syndrome in patients with essential thrombocythemia and polycythemia vera. Eur J Intern Med. 2017;41:49–54.
Mital A, Prejzner W, Bieniaszewska M, Hellmann A. Prevalence of acquired von Willebrand syndrome during essential thrombocythemia: a retrospective analysis of 170 consecutive patients. Pol Arch Med Wewn. 2015;125(12):914–20.
Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood. 2012;120(7):1409–11.
Finazzi G, Carobbio A, Thiele J, et al. Incidence and risk factors for bleeding in 1104 patients with essential thrombocythemia or prefibrotic myelofibrosis diagnosed according to the 2008 WHO criteria. Leukemia. 2012;26(4):716–9.
Tefferi A, Gangat N, Wolanskyj AP. Management of extreme thrombocytosis in otherwise low-risk essential thrombocythemia; does number matter? Blood. 2006;108(7):2493–4.
Tefferi A, Szuber N, Pardanani A, Hanson CA, Vannucchi AM, Barbui T, Gangat N. Extreme thrombocytosis in low-risk essential thrombocythemia: retrospective review of vascular events and treatment strategies. Am J Hematol. 2021;96(6):E182–4. https://doi.org/10.1002/ajh.26137.
Gangat N, Szuber N, Jawaid T, Hanson CA, Pardanani A, Tefferi A. Young platelet millionaires with essential thrombocythemia. Am J Hematol. 2021;96(4):E93–5.
Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol. 2012;156(2):281–4.
Galvez C, Stein BL. Thrombocytosis and thrombosis: is there really a correlation? Curr Hematol Malig Rep. 2020;15(4):261–7.
Gangat N, Wolanskyj AP, McClure RF, et al. Risk stratification for survival and leukemic transformation in essential thrombocythemia: a single institutional study of 605 patients. Leukemia. 2007;21(2):270–6.
Gisslinger H, Klade C, Georgiev P, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7(3):e196–208.
Mascarenhas JO, Prchal JT, Rambaldi A, et al. Interim analysis of the Myeloproliferative Disorders Research Consortium MPD-RC 112 global phase iii trial of front line pegylated interferon alpha-2a vs. hydroxyurea in high risk polycythemia vera and essential thrombocythemia. Blood. 2016;128(22):479.
Yacoub A, Mascarenhas J, Mesa RA, et al. Final results of prospective treatment with pegylated interferon alfa-2a for patients with polycythemia vera and essential thrombocythemia in first and second-line settings. Blood. 2019;1341(Supplement_1):2943.
How J, Hobbs G. Use of interferon alfa in the treatment of myeloproliferative neoplasms: perspectives and review of the literature. Cancers (Basel). 2020;12(7):1954. https://doi.org/10.3390/cancers12071954.
Daltro De Oliveira R, Soret-Dulphy J, Zhao L-P, et al. Interferon-alpha (IFN) therapy discontinuation is feasible in myeloproliferative neoplasm (MPN) patients with complete hematological remission. Blood. 2020;136(Supplement 1):35–6.
• Abu-Zeinah G, Krichevsky S, Cruz T, Hoberman G, Jaber D, Savage N, Sosner C, Ritchie EK, Scandura JM, Silver RT. Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia. 2021. https://doi.org/10.1038/s41375-021-01183-8. This retrospective study finds improved myelofibrosis-free and overall survival in PV patients treated with interferon-alpha compared to hydroxyurea or phlebotomy alone. Although prospective studies are necessary to validate these results, this study provides evidence challenging the current “watchful waiting” paradigm in low-risk PV patients.
• Barbui T, Vannucchi AM, De Stefano V, et al. Ropeginterferon alfa-2b versus phlebotomy in low-risk patients with polycythaemia vera (Low-PV study): a multicentre, randomised phase 2 trial. Lancet Haematol. 2021;8(3):e175–e184. https://doi.org/10.1016/S2352-3026(20)30373-2. This randomized phase 2 trial found a significant benefit with regard to hematocrit control in low-risk PV patients treated with interferon compared to phlebotomy alone. Treatment was well tolerated. This study provides rationale for early use of cytoreduction with interferon in low-risk PV patients.
Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer. 2016;122(3):477–85.
Pedersen KM, Zangger G, Brochmann N, et al. The effectiveness of exercise-based rehabilitation to patients with myeloproliferative neoplasms-an explorative study. Eur J Cancer Care (Engl). 2018;27(5):e12865.
Gowin K, Langlais BT, Kosiorek HE, et al. The SIMM study: survey of integrative medicine in myeloproliferative neoplasms. Cancer Med. 2020;9(24):9445–53.
Huberty J, Eckert R, Dueck A, et al. Online yoga in myeloproliferative neoplasm patients: results of a randomized pilot trial to inform future research. BMC Complement Altern Med. 2019;19(1):121.
Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76.
Diehn F, Tefferi A. Pruritus in polycythaemia vera: prevalence, laboratory correlates and management. Br J Haematol. 2001;115(3):619–21.
Baldo A, Sammarco E, Plaitano R, Martinelli V, Monfrecola A. Narrowband (TL-01) ultraviolet B phototherapy for pruritus in polycythaemia vera. Br J Dermatol. 2002;147(5):979–81.
Frewin R, Dowson A. Headache in essential thrombocythaemia. Int J Clin Pract. 2012;66(10):976–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Myeloproliferative Neoplasms
Rights and permissions
About this article
Cite this article
How, J., Hobbs, G. Management Issues and Controversies in Low-Risk Patients with Essential Thrombocythemia and Polycythemia Vera. Curr Hematol Malig Rep 16, 473–482 (2021). https://doi.org/10.1007/s11899-021-00649-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00649-x