Abstract
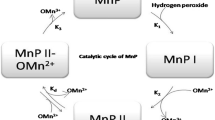
Lignin is the most abundant renewable source of aromatic polymer in nature, and its decomposition is indispensable for carbon recycling. It is chemically recalcitrant to breakdown by most organisms because of the complex, heterogeneous structure. The white-rot fungi produce an array of extracellular oxidative enzymes that synergistically and efficiently degrade lignin. The major groups of ligninolytic enzymes include lignin peroxidases, manganese peroxidases, versatile peroxidases, and laccases. The peroxidases are heme-containing enzymes with catalytic cycles that involve the activation by H2O2 and substrate reduction of compound I and compound II intermediates. Lignin peroxidases have the unique ability to catalyze oxidative cleavage of C–C bonds and ether (C–O–C) bonds in non-phenolic aromatic substrates of high redox potential. Manganese peroxidases oxidize Mn(II) to Mn(III), which facilitates the degradation of phenolic compounds or, in turn, oxidizes a second mediator for the breakdown of non-phenolic compounds. Versatile peroxidases are hybrids of lignin peroxidase and manganese peroxidase with a bifunctional characteristic. Laccases are multi-copper-containing proteins that catalyze the oxidation of phenolic substrates with concomitant reduction of molecular oxygen to water. This review covers the chemical nature of lignin substrates and focuses on the biochemical properties, molecular structures, reaction mechanisms, and related structures/functions of these enzymes.



















Similar content being viewed by others
References
Brunow, G. (2001). Methods to reveal the structure of lignin. In M. Hofrichter, & A. Steinbuchel (Eds.), Lignin, humic substances and coal (Biopolymers, vol. 1). Weinheim, Germany: Wiley-VCH.
Fukushima, K. (2001). Regulation of syringyl to guaiacyl ratio in lignin biosynthesis. Journal of Plant Research, 114, 499–508, doi:10.1007/PL00014017.
Higuchi, T. (2006). Look back over the studies of lignin biochemistry. Journal of Wood Science, 52, 2–8, doi:10.1007/s10086-005-0790-z.
Del Rio, J. C., Marques, G., Rencoret, J., Martinez, A. T., & Gutierrez, A. (2007). Occurrence of naturally acetylated lignin units. Journal of Agricultural and Food Chemistry, 55, 5461–5466, doi:10.1021/jf0705264.
Ralph, J., Lundquist, K., Brunow, G., Lu, F., Kim, H., Schatz, P. F., et al. (2004). Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochemistry Review, 3, 29–60, doi:10.1023/B:PHYT.0000047809.65444.a4.
Alder, E. (1977). Lignin chemistry - past, present and future. Wood Science and Technology, 11, 169–218, doi:10.1007/BF00365615.
Karhunen, P., Rummakko, P., Sipila, J., Brunow, G., & Kilpelainen, I. (1995). The formation of dibenzodiomocin structures by oxidative coupling. A model reaction for lignin biosynthesis. Tetrahedron Letter, 36, 4501–4504, doi:10.1016/0040-4039(95)00769-9.
Argyropoulos, D. S., Jurasek, L., Kristofova, L., Xia, Z. C., Sun, Y. J., & Palus, E. (2002). Abundance and reactivity of dibenzodioxocins in softwood lignin. Journal of Agricultural and Food Chemistry, 50, 658–666, doi:10.1021/jf010909g.
Zhang, L., & Gellerstedt, G. (2001). NMR observation of a new lignin structure, a spirodienone. Chemical Communications, 2744–2745.
Boerjan, W., Ralph, J., & Baucher, M. (2003). Lignin biosynthesis. Annual Review Plant Biology, 54, 519–546, doi:10.1146/annurev.arplant.54.031902.134938.
Davin, L. B., & Lewis, N. G. (2003). A historical perspective on lignan biosynthesis: Monolignol, allylphenol and hydroxycinnamic acid coupling and downstream metabolism. Phytochemistry Review, 2, 257–288, doi:10.1023/B:PHYT.0000046175.83729.b5.
Chen, Y.-R., & Sarkanen, S. (2003). Macromolecular lignin replication: A mechanistic working hypothesis. Phytochemistry Review, 2, 235–255, doi:10.1023/B:PHYT.0000046173.38194.ba.
Brauns, F. E. (1939). Native lignin I. Its isolation and methylation. Journal of the American Chemical Society, 61, 2120–2127, doi:10.1021/ja01877a043.
Buchanan, M. A., Brauns, F. E., & Leaf, Jr., R. L. (1949). Native lignin II. Native aspen lignin. Journal of the American Chemical Society, 71, 1297–1299, doi:10.1021/ja01172a043.
Bjorkman, A. (1954). Isolation of lignin from finely divided wood with neutral solvents. Nature, 174, 1057–1058, doi:10.1038/1741057a0.
Gellerstedt, G., Pranda, J., & Lindfors, E. L. (1994). Structural and molecular properties of residual birch kraft lignin. Journal of Wood Chemistry and Technology, 14, 467–482, doi:10.1080/02773819408003108.
Alder, E., Pepper, J. M., & Eriksoo, E. (1957). Action of mineral acid on lignin and model substances of guaiacylglycerol-beta-aryl ether type. Industrial Engineering Chemistry, 49, 1391–1392, doi:10.1021/ie50573a037.
Pew, J., & Weyna, P. (1962). Fine grinding, enzyme digestion and lignin-cellulose bond in wood. TAPPI, 45, 247–256.
Chang, H. M., Cowling, E. B., Brown, W., Alder, E., & Miksche, G. (1975). Comparative studies on cellulolytic enzyme lignin and milled wood lignin of Sweetgum and Spruce. Holzforschung, 29, 153–159.
Kirk, T. K., Connors, W. J., Bleam, R. D., Hackett, W. F., & Zeikus, J. G. (1975b). Preparation and microbial decomposition of synthetic [14C]lignins. Proceedings of the National Academy of Sciences of the USA, 72, 2515–2519, doi:10.1073/pnas.72.7.2515.
Kirk, T. K., & Brunow, G. (1988). Synthetic 14C-labeled lignins. Methods in Enzymology, 161, 65–73, doi:10.1016/0076-6879(88)61010-X.
Kirk, T. K., Connors, W. J., & Zeikus, G. (1976). Requirements for a growth substrate during lignin decomposition by two wood-rotting fungi. Applied and Environmental Microbiology, 32, 192–194.
Mester, T., Varela, E., & Tien, M. (2004). Wood degradation by brown-rot and white-rot fungi. The Mycota II: genetics and biotechnology (2nd edition). Springer-Verlag Berlin-Heidelberg.
Blanchette, R. A. (1984). Screening wood decayed by white rot fungi for preferential lignin degradation. Applied and Environmental Microbiology, 48, 647–653, Medline.
Otjen, L., & Blanchette, R. (1987). Assessment of 30 white rot basidiomycetes for selective lignin degradation. Holzforschung, 41, 343–349.
Blanchette, R. A. (1991). Delignification by wood-decay fungi. Annual Review of Phytopathology, 29, 381–398, doi:10.1146/annurev.py.29.090191.002121.
Eriksson, K.-E. L., Blanchette, R. A., & Ander, P. (1990). Microbial and Enzymatic Degradation of wood and wood components p. 407. Berlin Heidelberg: Springer-Verlag.
Gilbertson, R. L. (1980). Wood-rotting fungi of north America. Mycologia, 72, 1–49, doi:10.2307/3759417.
Cowling, E. B. (1961). Comparative biochemistry of decay of sweetgum sapwood by white-rot and brown-rot fungi. USDA Technical Bulletin, 1258, 1–79.
Baldrian, P. (2005). Fungal laccases - occurrence and properties. FEMS Microbiology Reviews, 30, 215–242, doi:10.1111/j.1574-4976.2005.00010.x.
Farrell, L. (1987). Combustion: The microbial degradation of lignin. Annual Reviews in Microbiology, 41, 465–505, doi:10.1146/annurev.micro.41.1.465.
Gold, H. M., Youngs, H. L., & Sollewijn Gelpke, M. D. (2000). Manganese peroxidase. Metal Ions in Biological Systems, 37, 559–586, Medline.
Kersten, P., & Cullen, D. (2007). Extracellular oxidative systems of the lignin-degrading Basidiomycete Phanerochaete chrysosporium. Fungal Genetics Biology, 44, 77–87, doi:10.1016/j.fgb.2006.07.007.
Martinez, A. T. (2002). Molecular biology and structure-function of lignin-degrading heme peroxidases. Enzyme Microbial Technology, 30, 425–444, doi:10.1016/S0141-0229(01)00521-X.
Martinez, A. T., Speranza, M., Ruiz-Duenas, F. J., Ferreira, P., Camarero, S., Guillen, F., et al. (2005). Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. International Microbiology, 8, 195–204, Medline.
Welinder, K. G. (1992). Superfamily of plant, fungal and bacterial peroxidases. Current Opinion in Structural Biology, 2, 388–393, doi:10.1016/0959-440X(92)90230-5.
Tien, M., & Kirt, T. K. (1983). Lignin-degrading enzyme from the hymenomycetes Phanerochaete chrysosporium burds. Science, 221, 661–663, doi:10.1126/science.221.4611.661.
Hammel, K. E., Jensen, Jr., K. A., Mozuch, M. D., Landucci, L. L., Tien, M., & Pease, E. A. (1993). Ligninolysis by a purified lignin peroxidase. Journal of Biological Chemistry, 268, 12274–12281, Medline.
Valli, K., Wariishi, H., & Gold, M. H. (1990). Oxidation of monomethoxylated aromatic compounds by lignin peroxidase: Role of veratryl alcohol in lignin biodegradation. Biochemistry, 29, 8535–8539, doi:10.1021/bi00489a005.
Edwards, S. L., Raag, R., Wariishi, H., Gold, M. H., & Poulos, T. L. (1993). Crystal structure of lignin peroxidase. Proceedings of the National Academy of Sciences of the USA, 90, 750–754, doi:10.1073/pnas.90.2.750.
Poulos, T. L., Edwards, S. L., Wariishi, H., & Gold, M. H. (1993). Crystallographic refinement of lignin peroxidase at 2Δ. Journal of Biological Chemistry, 268, 4429–4440, Medline.
Blodig, W., Smith, A. T., Doyle, W. A., & Piontek, K. (2001). Crystal structures of pristine and oxidatively processed lignin peroxidase expressed in Escherichia coli and of the W171F variant that eliminate the redox active tryptophan 171. Implications for the reaction mechanism. Journal of Molecular Biology, 305, 851–861, doi:10.1006/jmbi.2000.4346.
Choinowski, T., Blodig, W., Winterhalter, K. H., & Piontek, K. (1999). The crystal structure of lignin peroxidase at 1.70Δ resolution reveals a hydroxy group on the Cβ of tryptophan 171: A novel radical site formed during the redox cycle. Journal of Molecular Biology, 286, 809–827, doi:10.1006/jmbi.1998.2507.
Blodig, W., Doyle, W. A., Smith, A. T., Winterhalter, K., Choinowski, T., & Piontek, K. (1998). Autocatalytic formation of a hydroxy group at the Cβ of Trp171 in lignin peroxidase. Biochemistry, 37, 8832–8838, doi:10.1021/bi9727186.
Doyle, W. A., Blodig, W., Weitch, N. C., Piontek, K., & Smith, A. T. (1998). Two substrate interaction sites in lignin peroxidase revealed by site-directed mutangesis. Biochemistry, 37, 15097–15105, doi:10.1021/bi981633h.
Timofeevski, S. L., Nie, G., Reading, S., & Aust, S. D. (1999). Addition of veratryl alcohol oxidase activity to manganese peroxidase by site-directed mutagenesis. Biochemical and Biophysical Research Communications, 256, 500–504, doi:10.1006/bbrc.1999.0360.
Timofeevski, S. L., Nie, G., Reading, S., & Aust, S. D. (2000). Substrate specificity of lignin peroxidase and a S168W variant of manganese peroxidase. Archives of Biochemistry and Biophysics, 373, 147–153, doi:10.1006/abbi.1999.1562.
Wong, D. W. S. (1995). Food enzymes: structure and mechanism pp. 321–345. NY: Chapman & Hall.
Renganathan, V., & Gold, M. H. (1986). Spectral characterization of the oxidized states of lignin peroxidase, an extracellular heme enzyme from the white rot Basidiomycete Phanerochaete chrysosporium. Biochemistry, 25, 1626–1631, doi:10.1021/bi00355a027.
Andrawis, A., Johnson, K. A., & Tien, M. (1988). Studies on Compound I formation of the lignin peroxidase from Phanerochaete chrysosporium. Journal of Biological Chemistry, 263, 1196–1198.
Marquez, L., Wariishi, H., Dunford, H. B., & Gold, M. H. (1988). Spectroscopic and kinetic properties of the oxidized intermediates of lignin peroxidase from Phanerochaete chrysosporium. Journal of Biological Chemistry, 263, 10549–10552, Medline.
Behere, D. V., Gonzalez-Vergara, E., & Goff, H. M. (1985). Unique cyanide nitrogen-15 nuclear magnetic resonance chemical shift values for cyano-eroxidase complexes. Relevance to the heme active-site structure and mechanism of peroxide activation. Biochimica et Biophysica Acta, 832, 319–325.
Chance, B., Power, L., Ching, Y., Poulos, T., Schonbaum, G. R., Yamazaki, I., & Paul, K. G. (1984). X-Ray absorption studies of intermediates in peroxidase activity. Archives of Biochemistry and Biophysics, 235, 596–611, doi:10.1016/0003-9861(84)90234-0.
Blodig, W., Smith, A. T., Winterhalter, K., & Piontek, K. (1999). Evidence from spin-trapping for a transient radical on tryptophan residue 171 of lignin peroxidase. Archives of Biochemistry and Biophysics, 370, 86–92, doi:10.1006/abbi.1999.1365.
Tien, M., Kirk, T. K., Bull, C., & Fee, J. A. (1986). Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by the ligninase of Phanerocheate chrysosporium Burds. Journal of Biological Chemistry, 261, 1687–1683, Medline.
Ator, M. A., & de Montelano, P. R. O. (1987). Protein control of prosthetic heme activity. Reaction of substrates with the heme edge of horseradish peroxidase. Journal of Biological Chemistry, 262, 1542–1551, Medline.
Schoemaker, H. E., & Piontek, K. (1996). On the interaction of lignin peroxidase with lignin. Pure and Applied Chemistry, 68, 2089–2096, doi:10.1351/pac199668112089.
Sollewijn Gelpke, M. D., Lee, J., & Gold, M. H. (2002). Lignin peroxidase oxidation of veratryl alcohol: Effects of the mutants H82A, Q222A, W171A, and F267L. Biochemistry, 41, 3498–3506, doi:10.1021/bi011930d.
Johjima, T., Wariishi, H., & Tanaka, H. (2002). Veratryl alcohol binding sites of lignin peroxidase from Phanerochaete chrysosporium. Journal of Molecular Catalysis. B, Enzymatic, 17, 49–57.
Gerini, M. F., Roccatano, D., Baciocchi, E., & Nola, A. D. (2003). Molecular dynamics simulations of lignin peroxidase in solution. Biophysical Journal, 84, 3883–3893.
Wariishi, H., & Gold, M. H. (1989). Lignin peroxidase compound III. Formation, inactivation, and conversion to the native enzyme. FEBS Letters, 243, 165–168, doi:10.1016/0014-5793(89)80122-X.
Cai, D., & Tien, M. (1992). Kinetic studies on the formation and decomposition of compounds II and III. Journal of Biological Chemistry, 267, 1149–1155.
Barr, D. P., & Aust, S. D. (1994). Conversion of lignin peroxidase compound III to active enzyme by cation radicals. Archives of Biochemistry and Biophysics, 312, 511–515, doi:10.1006/abbi.1994.1339.
Schoemaker, H. E., Harvey, P. J., Bowen, R. M., & Palmer, J. M. (1985). On the mechanism of enzymatic lignin breakdown. FEBS Letters, 183, 7–12, doi:10.1016/0014-5793(85)80942-X.
Baciocchi, E., Gerini, M. F., Lanzalunga, O., Lapi, A., Piparo, M. G. L., & Mancinelli, S. (2001a). Isotope-effect profiles in the oxidative N-demethylation of N,N-dimethylanilines catalyzed by lignin peroxidase and a chemical model. European Journal of Organ Chemistry, 2001, 2305–2310, doi:10.1002/1099-0690(200106)2001:12<2305::AID-EJOC2305>3.0.CO;2-E.
Harvey, P. J., & Palmer, J. M. (1990). Oxidation of phenolic compounds by ligninase. Journal of Biotechnology, 13, 169–179, doi:10.1016/0168-1656(90)90102-H.
Tien, M., & Kirk, T. K. (1984). Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proceedings of the National Academy of Sciences of the USA, 81, 2280–2284, doi:10.1073/pnas.81.8.2280.
Harvey, G.-F., Gilardi, G.-F., Goble, M. L., & Palmer, J. M. (1993). Charge transfer reactions and feedback control of lignin peroxidase by phenolic compounds: Significance in lignin degradation. Journal of Biotechnology, 30, 57–69, doi:10.1016/0168-1656(93)90027-K.
Koduri, R. S., & Tien, M. (1995). Oxidation of guaiacol by lignin peroxidase. Journal of Biological Chemistry, 270, 22254–22258, doi:10.1074/jbc.270.38.22254.
Dodson, P. J., Evans, C. S., Harvey, P. J., & Palmer, J. M. (1987). Production and properties of an extracellular peroxidase from Corilus versicolor which catalyzes \({\text{C}}_\alpha {\text{ - C}}_\beta \) cleavage in a lignin model compound. FEMS Microbiology Letter, 42, 17–22.
Chung, N., & Aust, S. D. (1995). Inactivation of lignin peroxidase by hydrogen peroxide during the oxidation of phenols. Archives of Biochemistry and Biophysics, 316, 851–855, doi:10.1006/abbi.1995.1114.
Banci, L., Ciofi-Baffoni, S., & Tien, M. (1999). Lignin and Mn peroxidase-catalyzed oxidation of phenolic lignin oligomers. Biochemistry, 38, 3205–3210, doi:10.1021/bi982139g.
Renganathan, V., Miki, K., & Gold, M. H. (1985). Multiple molecular forms of diarylpropane oxygenase, an H2O2-requiring, lignin-degrading enzyme from Phanerochate chrysosporium. Archives of Biochemistry and Biophysics, 241, 304–314, doi:10.1016/0003-9861(85)90387-X.
Hammel, K. E., Tien, M., Kalyanaraman, B., & Kirk, T. K. (1985). Mechanism of oxidative \({\text{C}}_\alpha - {\text{C}}_\beta \) cleavage of a lignin model dimer by Phanerochaete chrysosporium ligninase. Journal of Biological Chemistry, 260, 8348–8353.
Kirk, T. K., Tien, M., Kersten, P. J., & Mozuch, M. D. (1986). Ligninase of Phanerochaete chrysosporium. Biochemistry Journal, 236, 279–287.
Miki, K., Renganathan, V., & Gold, M. H. (1986). Mechanism of β-aryl ether dimeric lignin model compound oxidation by lignin peroxidase of Phanerochaete chrysosporium. Biochemistry, 25, 4790–4796, doi:10.1021/bi00365a011.
Miki, K., Kondo, R., Renganathan, V., Mayfield, M. B., & Gold, M. H. (1988). Mechanism of aromatic ring cleavage of a β-biphenylyl ether dimer catalyzed by lignin peroxidase of Phanerochaete chrysosporium. Biochemistry, 27, 4787–4794, doi:10.1021/bi00413a031.
Umezawa, T., & Higuchi, T. (1989). Cleavages of aromatic ring and β-O-4 bond of synthetic lignin (DHP) by lignin peroxidase. FEB Letter, 242, 325–329, doi:10.1016/0014-5793(89)80494-6.
Kersten, P. J., Tien, M., Kalyanaraman, B., & Kirk, T. K. (1985). The ligninase of Phanerochaete chrysosporium generates cation radicals from methoxybenzenes. Journal of Biological Chemistry, 260, 2609–2612.
Baciocchi, E., Bietti, M., Gerini, M. F., Lanzalunga, O., & Mancinelli, S. (2001b). Oxidation of non-phenolic β-O-aryl-lignin model dimers catalyzed by lignin peroxidase. Comparison with the oxidation induced by potassium 12-tungstocobalt(III)ate. Journal of the Chemical Society, 41, 1506–1511, doi:10.1039/b101362i, Perkin Trans 2.
Lundell, T., Wever, R., Floris, R., Harvey, P., Hatakka, A., Brunow, G., et al. (1993). Lignin peroxidase L3 from Phlebia radiata. Pre-steady-state and steady-state studies with veratryl alcohol and a non-phenolic lignin model compound 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)propane-1,3-diol. European Journal of Biochemistry, 211, 391–402, doi:10.1111/j.1432-1033.1993.tb17562.x.
Harvey, P. J., Schoemaker, H. E., Bowen, R. M., & Palmer, J. M. (1985). Single-electron transfer processes and the reaction mechanism of enzymic degradation of lignin. FEBS Letters, 183, 13–16, doi:10.1016/0014-5793(85)80943-1.
Wariishi, H., Huang, J., Dunford, B., & Gold, M. H. (1991). Reactions of lignin compounds I and II with veratryl alcohol. Journal of Biological Chemistry, 266, 20694–20699.
Walling, C., El-Talwi, G. M., & Amarnalth, K. (1984). Oxidation of styrene derivatives by S2O8 2–CuII in acetic acid and acetonitrile. Reaction paths in oxidations via radical cations. Journal of the American Chemical Society, 106, 7373–7578, doi:10.1021/ja00336a043.
Fenn, P., & Kirk, T. K. (1981). Relationship of nitrogen to the onset and suppression of ligninolytic activity and secondary metabolism in Phanerochaete chrysosporium. Archives of Microbiology, 130, 59–65, doi:10.1007/BF00527073.
Faison, B. D., & Kirk, T. K. (1985). Factors involved in the regulation of ligninase activity in Phanerochaete chrysosporium. Applied and Environmental Microbiology, 49, 299–304.
Schoemaker, H. E., & Leisola, M. S. A. (1990). Degradation of lignin by Phanerochaete chrysosporium. Journal of Biotechnology, 13, 101–109, doi:10.1016/0168-1656(90)90096-T.
Bietti, M., Baciocchi, E., & Steenken, S. (1998). Lifetime, reduction potential and base-induced fragmentation of the veratryl alcohol radical cation in aqueous solution. Pulse radiolysis studies on a ligninase “mediator”. Journal of Physical Chemistry A, 102, 7337–7342, doi:10.1021/jp9812482.
Harvey, P. J., Schomaker, H. E., & Palmer, J. M. (1986). Veratryl alcohol as a mediator and the role of radical cations in lignin biodegradation by Phanerochaete chrysosporium. FEBS Letters, 195, 242–246, doi:10.1016/0014-5793(86)80168-5.
Khindaria, A., Grover, T. A., & Aust, S. D. (1995). Evidence for formation of the veratryl alcohol cation radical by lignin peroxidase. Biochemistry, 34, 6020–6025, doi:10.1021/bi00018a003.
Khindaria, A., Nie, G., & Aust, S. D. (1997). Detection and characterization of the lignin peroxidase compound II - veratryl alcohol cation radical complex. Biochemistry, 36, 14181–14185 doi:10.1021/bi9715730.
Baciocchi, E., Bietti, M., Gerini, M. F., & Lanzalunga, O. (2002). The mediation of veratryl alcohol in oxidations prompted by lignin peroxidase: the lifetime of veratryl alcohol radical cation. Biochemistry and Biophysics Research Communications, 293, 832–835, doi:10.1016/S0006-291X(02)00306-6.
Schmidt, H. W. H., Haemmerli, S. D., Schoemaker, H. E., & Leisola, M. S. A. (1989). Oxidative degradation of 3,4-dimethoxybenzyl alcohol and its methyl ether by the lignin peroxidase of Phanerochaete chrysosporium. Biochemistry, 28, 1776–1783, doi:10.1021/bi00430a053.
Haemmerli, S. D., Schoemaker, H. E., Schmidt, H. W. H., & Leisola, M. S. A. (1987). Oxidation of veratryl alcohol by the lignin peroxidase of Phanerochaete chrysosporium. Involvement of activated oxygen. FEBS Letters, 220, 149–154, doi:10.1016/0014-5793(87)80893-1.
Have, R. T., & Franssen, M. C. R. (2001). On a revised mechanism of side product formation in the lignin peroxidase catalyzed oxidation of veratryl alcohol. FEBS Letters, 487, 313–317 doi:10.1016/S0014-5793(00)02379-6.
Goodwin, D. C., Aust, S. D., & Grover, T. A. (1995). Evidence for veratryl alcohol as a redox mediator in lignin peroxidase-catalyzed oxidation. Biochemistry, 34, 5060–5065, doi:10.1021/bi00015a017.
Tien, M., & Ma, D. (1997). Oxidation of 4-methoxymandelic acid by lignin peroxidase. Journal of Biological Chemistry, 272, 8912–8917, doi:10.1074/jbc.272.14.8912.
Christian, V., Shrivastava, R., Shukla, D., Modi, H., & Vyas, B. R. M. (2005). Mediator role of veratryl alcohol in the lignin peroxidase-catalyzed oxidative decolorization of remazol brilliant blue R. Enzyme Microbial Technology, 36, 426–431, doi:10.1016/j.enzmictec.2004.06.007.
Khindaria, A., Yamazaki, I., & Aust, S. D. (1996). Stabilization of the veratryl alcohol cation radical by lignin peroxidase. Biochemistry, 35, 6418–6424, doi:10.1021/bi9601666.
Baciocchi, E., Bietti, M., & Steenken, S. (1998). Lifetime, reduction potential and base-induced fragmentation of the veratryl alcohol radical cation in aqueous solution. Pulse radiolysis studies on a ligninase “mediator”. Journal of Physical Chemistry A, 102, 7337–7342, doi:10.1021/jp9812482.
Joshi, D. K., & Gold, M. H. (1996). Oxidation of dimethoxylated aromatic compounds by lignin peroxidase from Phanerochaete chrysosporium. European Journal of Biochemistry, 237, 45–57, doi:10.1111/j.1432-1033.1996.0045n.x.
Kersten, P. J., Kalyanaraman, B., Hammel, K. E., & Reinhammar, B. (1990). Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzene. Biochemistry Journal, 268, 475–480.
Cui, F., & Dolphin, D. (1990). The role of manganese in model systems related to lignin biodegradation. Holzforschung, 44, 279–283.
Reinhammar, B. R. M. (1972). Oxidation-reduction potentials of the electron acceptors in laccase and stellacyanin. Biochimica et Biophysica Acta, 275, 245–259, doi:10.1016/0005-2728(72)90045-X.
Xu, F., Berka, R. M., Wahleithner, J. A., Nelson, B. A., Shuster, J. R., Brown, S. H., et al. (1998). Site-directed mutations in fungal laccase: effect on redox potential, activity and pH profile. Biochemistry Journal, 334, 63–70.
Hayashi, Y., & Yamazaki, I. (1979). The oxidation-reduction potentials of compound I/compound II and compound II/ferric couples of horseradish peroxidase A2 and C. Journal of Biological Chemistry, 254, 9101–9106.
Conroy, C. W., Tyma, P., Daum, P. H., & Erman, J. E. (1978). Oxidation-reduction potential measurements of cytochrome c peroxidase and pH dependent spectral transition in the ferrous enzyme. Biochimica et Biophysica Acta, 537, 62–69.
Popp, J. L., & Kirk, T. K. (1991). Oxidation of methoxybenzenes by manganese peroxidase and by Mn3+. Archives of Biochemistry and Biophysics, 268, 145–148, doi:10.1016/0003-9861(91)90176-J.
Bonnarme, P., & Jeffries, T. W. (1990). Mn(II) regulation of lignin peroxidases and manganese-dependent peroxidases from lignin-degrading white rot fungi. Applied Environmental Microbiology, 56, 210–217.
Brown, J. A., Alic, M., & Gold, M. H. (1991). Manganese peroxidase gene transcription in Phanerochaete chrysosporium: Activation by manganese. Journal of Bacteriology, 173, 4101–4106.
Gettemy, J. M., Ma, B., Alic, M., & Gold, M. H. (1998). Reverse transcription-PCR analysis of the regulation of the manganese peroxidase gene family. Applied and Environmental Microbiology, 64, 569–574.
Alic, M., Akileswaran, L., & Gold, M. H. (1997). Characterization of the gene encoding manganese peroxidase isozyme 3 from Phanerochaete chrysosporium. Biochimica et Biophysica Acta, 1338, 1–7.
Mester, T., & Field, J. A. (1997). Optimization of manganese peroxidase production by the white rot fungus Bjerkandera sp. strain BOS55. FEMS Microbiology Letter, 155, 161–168, doi:10.1111/j.1574-6968.1997.tb13873.x.
Mester, T., de Jong, E., & Field, J. A. (1995). Manganese regulation of veratryl alcohol in white rot fungi and its indirect effect on lignin peroxidase. Applied and Environmental Microbiology, 61, 1881–1887.
Pease, E. A., & Tien, M. (1992). Heterogeneity and regulation of manganese peroxidases from Phanerochaete chrysosporium. Journal of Bacteriology, 174, 3532–3540.
Glenn, J. K., & Gold, M. H. (1985). Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading Basidiomycete, Phanerochaete chrysosporium. Archives of Biochemistry and Biophysics, 242, 329–341, doi:10.1016/0003-9861(85)90217-6.
Mino, Y., Wariishi, H., Blackburn, N. J., Loehr, T. M., & Gold, M. H. (1988). Spectral characterization of manganese peroxidase, an extracellular heme enzyme from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Journal of Biological Chemistry, 263, 7029–7036.
Sundaramoorthy, M., Kishi, K., Gold, M. H., & Poulos, T. L. (1994). The crystal structure of manganese peroxidase from Phanerochaete chrysosporium at 2.06-Δ resolution. Journal of Biological Chemistry, 269, 32759–32767.
Sundaramoorthy, M., Kishi, K., Gold, M. H., & Poulos, T. L. (1997). Crystal structure of substrate binding site mutants of manganese peroxidase. Journal of Biological Chemistry, 272, 17574–17580, doi:10.1074/jbc.272.28.17574.
Sundaramoorthy, M., Youngs, H. L., Gold, M. H., & Poulos, T. L. (2005). High-resolution crystal structure of manganese peroxidase: Substrate and inhibitor complexes. Biochemistry, 44, 6463–6470, doi:10.1021/bi047318e.
Glenn, J. K., Akileswaran, L., & Gold, M. H. (1986). Mn(II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium. Archives of Biochemistry and Biophysics, 251, 688–696, doi:10.1016/0003-9861(86)90378-4.
Paszczynski, A., Huynh, V. -B., & Crawford R. (1986). Comparison of ligninase-I and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. 244, 750–765.
Wariishi, H., Dunford, H. B., MacDonald, I. D., & Gold, M. H. (1989a). Manganese peroxidase from the lignin-degrading Basidiomycete Phanerochaete chrysosporium. Transient state kinetics and reaction mechanism. Journal of Biological Chemistry, 264, 3335–3340.
Wariishi, H., Akileswaran, L., & Gold, M. H. (1988). Manganese peroxidase from the Basidiomycete Phanerochaete chrysosporium: Spectral characterization of the oxidized states and the catalytic cycle. Biochemistry, 27, 5365–5370, doi:10.1021/bi00414a061.
Wariishi, H., Valli, K., & Gold, M. H. (1992). Manganese(II) oxidation by manganese peroxidase from the Basidiomycete Phanerochaete chrysosporium. Journal of Biological Chemistry, 267, 23688–23695.
Kishi, K., Wariishi, H., Marquez, L., Dunford, H. B., & Gold, M. H. (1994). Mechanism of manganese peroxidase compound II reduction. Effect of organic acid chelators and pH. Biochemistry, 33, 8694–8701, doi:10.1021/bi00195a010.
Kuan, I.-C., Johnson, K. A., & Tien, M. (1993). Kinetic analysis of manganese peroxidase. Journal of Biological Chemistry, 268, 20064–20070.
Zapanta, L. S., & Tien, M. (1997). The roles of veratryl alcohol and oxalate in fungal lignin degradation. Journal of Biotechnology, 53, 93–102, doi:10.1016/S0168-1656(96)01678-1.
Khindaria, A., Grover, T. A., & Aust, S. D. (1994). Oxalate-dependent reductive activity of manganese peroxidase from Phanerochaete chrysosporium. Archives of Biochemistry and Biophysics, 314, 301–306, doi:10.1006/abbi.1994.1446.
Urzua, U., Kersten, P. J., & Vicuna, R. (1998). Manganese peroxidase-dependent oxidation of glyoxylic and oxalic acids synthesized by Ceriporiopsis subvermispora produces extracellular hydrogen peroxide. Applied and Environmental Microbiology, 64, 68–73.
Wariishi, H., Valli, K., Renganathan, V., & Gold, M. H. (1989b). Oxidative cleavage of a phenolic diarylpropane lignin model dimer by manganese peroxidase from Phanerochaete chrysosporium. Biochemistry, 28, 6017–6023, doi:10.1021/bi00440a044.
Tuor, U., Wariishi, H., Schoemaker, H. E., & Gold, M. H. (1992). Oxidation of phenolic aryglycerol β-aryl ether lignin model compounds by manganese peroxidase from Phanerochaete chrysosporium: Oxidative cleavage of an α-carbonyl model compound. Biochemistry, 31, 4986–4995, doi:10.1021/bi00136a011.
Reddy, G. V. B., Sridhar, M., & Gold, M. H. (2003). Cleavage of nonphenolic β-1 diarylpropane lignin model dimers by manganese peroxidase from Phanerochaete chrysosporium. European Journal of Biochemistry, 270, 284–292, doi:10.1046/j.1432-1033.2003.03386.x.
Wariishi, H., Valli, K., Renganathan, V., & Gold, M. H. (1989c). Thiol-mediated oxidation of nonphenolic lignin model compounds by manganese peroxidase of Phanerochaete chrysosporium. Journal of Biological Chemistry, 264, 14185–14191.
Bao, W., Fukushima, Y., Jensen, K. A., Moen, M. A., & Hammel, K. E. (1994). Oxidative degradation of non-phenolic lignin during lipid peroxidation by fungal manganese peroxidase. FEBS Letters, 354, 297–300, doi:10.1016/0014-5793(94)01146-X.
Daina, S., Orlandi, M., Bestetti, G., Wiik, C., & Elegir, G. (2002). Degradation of β-5 lignin model dimers by Ceriporiopsis subvermispora. Enzyme Microbial Technology, 30, 499–505, doi:10.1016/S0141-0229(01)00524-5.
Kapich, A., Hofrichter, M., Vares, T., & Hatakka, V. (1999). Coupling of manganese peroxidase-mediated lipid peroxidation with destruction of nonphenolic lignin model compounds and 14C-labeled lignins. Biochemical and Biophysical Research Communications, 259, 212–219, doi:10.1006/bbrc.1999.0742.
Kapich, A. N., Steffen, K. T., Hofrichter, M., & Hatakka, A. (2005). Involvement of lipid oxidation in the degradation of a non-phenolic lignin model compound by manganese peroxidase of the litter-decomposing fungus Stropharia coronilla. Biochemical and Biophysical Research Communications, 330, 371–377, doi:10.1016/j.bbrc.2005.02.167.
Wariishi, H., & Gold, M. H. (1990). Lignin peroxidase compound III. Mechanism of formation and decomposition. Journal of Biological Chemistry, 265, 2070–2077.
Timofeevski, S. L., Reading, N. S., & Aust, S. D. (1998). Mechanisms for protection against inactivation of manganese peroxidase by hydrogen peroxide. Archives of Biochemistry and Biophysics, 356, 287–295, doi:10.1006/abbi.1998.0776.
Yoshida, H. (1883). Chemistry of lacquer (urushi). Journal of the Chemical Society, 43, 472–486.
Givaudan, A., Effosse, A., Faure, D., Potier, P., Bouillant, M.-L., & Bally, R. (1993). Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: Evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiology Letter, 108, 205–210, doi:10.1111/j.1574-6968.1993.tb06100.x.
Suzuki, T., Endo, K., Ito, M., Tsujibo, H., Miyamoto, K., & Inamori, Y. (2003). A thermostable laccase from Streptomyces lavendulae REN-7: Purification, characterization, nucleotide sequence, and expression. Bioscience, Biotechnology, and Biochemistry, 67, 2167–2175, doi:10.1271/bbb.67.2167.
Hullo, M.-F., Moszer, I., Danchin, A., & Martin-Verstraete, I. (2001). CotA of Bacillus subtilis is a copper-dependent laccase. Journal of Bacteriology, 183, 5426–5430, doi:10.1128/JB.183.18.5426-5430.2001.
Marco, A. E., & Roubelakis-Angelakis, K. A. (1997). Laccase activity could contribute to cell-wall reconstitution in regenerating protoplasts. Phytochemistry, 46, 421–425, doi:10.1016/S0031-9422(97)00301-4.
Bao, W., O’Malley, D. M., Whetten, R., & Sederoff, R. R. (1993). A laccase associated with lignification in Loblolly pine xylem. Science, 672–674, doi:10.1126/science.260.5108.672.
Sterjiades, R., Dean, J. F. D., Gamble, G., Himmelsbach, D. S., & Eriksson, K. -E. L. (1993). Extracellular laccases and peroxidases from sycamore mapple (Acer psudoplatanus) cell-suspension cultures. Planta, 190, 75–87, doi:10.1007/BF00195678.
Leatham, G. F., & Stahmann, M. A. (1981). Studies on the laccase of Lentinus edodes: specificity, localization and association with the development of fruiting bodies. Journal of General Microbiology, 125, 147–157.
Youn, H.-D., Hah, Y. C., & Kang, S.-O. (1995). Role of laccase in lignin degradation by white-rot fungi. FEMS Microbiology Letter, 132, 183–188, doi:10.1111/j.1574-6968.1995.tb07831.x.
Eggert, C., Temp, U., & Eriksson, K. -E. L. (1997). Laccase is essential for lignin degradation by the white-rot fungus Pycnoporus cinnabarinus. FEBS Letters, 407, 89–92, doi:10.1016/S0014-5793(97)00301-3.
Thurston, C. F. (1994). The structure and function of fungal laccases. Microbiology, 140, 19–26.
Bertrand, T., Jolivalt, C., Briozzo, P., Caminade, E., Joly, N., Madzak, C., et al. (2002). Crystal structure of a four-copper laccase complex with an arylamine: Insights into substrate recognition and correlation with kinetics. Biochemistry, 41, 7325–7333, doi:10.1021/bi0201318.
Ducros, V., Brzozowski, A. M., Wilson, K. S., Brown, S. H., Ostergaard, P., Schneider, P., et al. (1998). Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2Δ resolution. Nature Structural Biology, 5, 310–316, doi:10.1038/nsb0498-310.
Piontek, K., Antorini, M., & Choinowski, T. (2002). Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Δ resolution containing a full complement of coppers. Journal of Biological Chemistry, 277, 37663–37669, doi:10.1074/jbc.M204571200.
Hakulinen, N., Kiiskinen, L.-L., Kruus, K., Saloheimo, M., Paananen, A., Koivula, A., et al. (2002). Crystal structure of a laccase of Melanocarpus albomyces with an intact trinuclear copper site. Nature Structural Biology, 9, 601–605.
Antorini, M., Herpoel-Gimbert, I., Choinowski, T., Sigoillot, J.-C., Asther, M., Winterhalter, K., et al. (2002). Purification, crystallization and X-ray diffraction study of fully functional laccases from two ligninolytic fungi. Biochimica et Biophysica Acta, 1594, 109–114.
Garavaglia, S., Cambria, M. T., Miglio, M., Ragusa, S., Lacobazzi, V., Palmieri, F., et al. (2004). The structure of Rigidoporus lignosus laccase containing a full component of copper ions, reveals an asymmetrical arrangement for the T3 copper pair. Journal of Molecular Biology, 342, 1519–1531, doi:10.1016/j.jmb.2004.07.100.
Enguita, F. J., Martins, L. O., Henriquest, A. O., & Carrondo, M. A. (2003). Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. Journal of Biological Chemistry, 278, 19416–19425, doi:10.1074/jbc.M301251200.
Malkin, R., & Malmstrom, B. G. (1970). The state and function of copper in biological systems. Advance Enzymology, 33, 177.
Dooley, D. M., Rawlings, J., Dawson, J. H., Stephens, P. J., Andreasson, L.-E., Malmstrom, B. C., et al. (1979). Spectroscopic studies of Rhus vernicifera and Polyporus versicolor laccase. Electronic structures of the copper sites. Journal of the American Chemical Society, 101, 5038–5046, doi:10.1021/ja00511a039.
Palmer, A. E., Randall, D. W., Xu, F., & Solomon, E. I. (1999). Spectroscopic studies and electronic structure description of the high potential type 1 copper site in fungal laccase: Insight into the effect of the axial ligand. Journal of the American Chemical Society, 121, 7138–7149, doi:10.1021/ja991087v.
Reinhammar, B. R. M., & Vanngard, T. I. (1971). The electron-accepting sites in Rhus vernicifera laccase as studied by anaerobic oxidation-reduction titrations. European Journal of Biochemistry, 18, 463–468, doi:10.1111/j.1432-1033.1971.tb01264.x.
Xu, F., Shin, W., Brown, S. H., Wahleithner, J. A., Sundaram, U. M., & Solomon, E. I. (1996). A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochimica et Biophysica Acta, 1292, 303–311.
Xu, F. (1996). Oxidation of phenols, anilines, and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry, 35, 7608–7614, doi:10.1021/bi952971a.
Messerschmidt, A., Ladenstein, R., Huber, R., Bolognesi, M., Avigliano, L., Petruzzelli, R., et al. (1992). Refined crystal structure of ascorbate oxidase at 1.9Δ resolution. Journal of Molecular Biology, 224, 179–205, doi:10.1016/0022-2836(92)90583-6.
Zoppellaro, G., Sakurai, T., & Huang, H.-W. (2001). A novel mixed valence form of Rhus vernicifera laccase and its reaction with dioxygen to give a peroxide intermediate bound to the trinuclear center. Journal of Biochemistry, 129, 949–953.
Huang, H.-W., Zopellaro, G., & Sakurai, T. (1999). Spectroscopic and kinetic studies on the oxygen-centered radical formed during the four-electron reduction process of dioxygen by Rhus vernicifera laccase. Journal of Biological Chemistry, 274, 32718–32724, doi:10.1074/jbc.274.46.32718.
Palmer, A. E., Lee, S. K., & Solomon, E. I. (2001). Decay of the peroxide intermediate in laccase: Reductive cleavage of the O-O bond. Journal of the American Chemical Society, 123, 6591–6599, doi:10.1021/ja010365z.
Lee, S.-K., George, S. D., Antholine, W. E., Hedman, B., Hodgson, K. O., & Solomon, E. I. (2002). Nature of the intermediate formed in the reduction of O2 to H2O at the trinuclear copper cluster active site in native laccase. Journal of the American Chemical Society, 124, 6180–6193, doi:10.1021/ja0114052.
Zoppellaro, G., Huang, H.-W., & Sakurai, T. (2000). Kinetic studies on the reaction of the fully reduced laccase with dioxygen. Inorganic Reaction Mechanisms, 2, 79–84.
Lundquist, K., & Kristersson, P. (1985). Exhaustive laccase-catalyzed oxidation of a lignin model compound (vanillyl glycol) produces methanol and polymeric quinoid products. Biochemistry Journal, 259, 277–279.
Kawai, S., Umezawa, T., & Higuchi, T. (1999a). Degradation mechanisms of phenolic β-1 lignin substructure model compounds by laccase of Coriolus versicolor. Archives of Biochemistry and Biophysics, 262, 99–110, doi:10.1016/0003-9861(88)90172-5.
Faure, D., Bouillant, M. L., Jacoud, C., & Bally, R. (1996). Phenolic derivatives related to lignin metabolism as substrates for Azospirillum laccase activity. Phytochemistry, 42, 357–359, doi:10.1016/0031-9422(95)00869-1.
Kawai, S., Umezawa, T., Shimada, M., & Higuchi, T. (1999b). Aromatic ring cleavage of 4,6-di(tert)guaiacol, a phenolic lignin model compound, by laccause of Coriolus versicolor. FEBS Letters, 236, 309–311, doi:10.1016/0014-5793(88)80043-7.
Bourbonnais, R., & Paice, M. G. (1990). Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. 267, 99–102.
Bourbonnais, R., Paice, M. G., Freiermuth, B., Bodie, E., & Borneman, S. (1997). Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Applied and Environmental Microbiology, 63, 4627–4632.
Eggert, C., Temp, U., Dean, J. F. D., & Eriksson, K.-E. L. (1996). A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Letters, 391, 144–148, doi:10.1016/0014-5793(96)00719-3.
Fabbrini, M., Galli, C., & Gentili, P. (2002). Comparing the catalytic efficiency of some mediators of laccase. Journal of Molecular Catalysis. B, Enzymatic, 16, 231–240, doi:10.1016/S1381-1177(01)00067-4.
Baiocco, P., Barreca, A. M., Fabbrini, M., Galli, C., & Gentili, P. (2003). Promoting laccase activity towards non-phenolic substrates: a mechanistic investigation with some laccase-mediator systems. Organ Biomolecular Chemistry, 1, 191–197, doi:10.1039/b208951c.
Bourbonnais, R., Leech, D., & Paice, M. G. (1998). Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochimica et Biophysica Acta, 1379, 381–390.
Claus, H., Faber, G., & Konig, H. (2002). Redox-mediated decolorization of synthetic dyes by fungal laccases. Applied Microbiology and Biotechnology, 59, 672–678, doi:10.1007/s00253-002-1047-z.
Gutierrez, A., del Rio, J. C., Ibarra, D., Rencoret, J., Romero, J., Speranza, M., et al. (2006a). Enzymatic removal of free and conjugated sterols forming pitch deposits in environmentally sound bleaching of eucalypt paper pulp. Environmental Science & Technology, 40, 3416–3422, doi:10.1021/es052547p.
Gutierrez, A., del Rio, J. C., Rencoret, J., Ibarra, D., & Martinez, A. T. (2006b). Main liphilic extractives in different paper pulp tpes can be removed using the laccase-mediator system. Applied Microbiology and Biotechnology, 72, 845–851, doi:10.1007/s00253-006-0346-1.
Gutierrez, A., Rencoret, J., Ibarra, D., Molina, S., Camareo, S., Romero, J., et al. (2007). Removal of lipophilic extractives from paper by laccase and lignin-derived phenols as natural mediators. Environmental Science & Technology, 41, 4124–4129, doi:10.1021/es062723+.
Kawai, S., Nakagawa, M., & Ohashi, H. (2002). Degradation mechanisms of nonphenolic β-O-4 lignin model dimer by Trametes versicolor laccase in the presence of 1-hydroxybenzotriazole. Enzyme Microbial Technology, 30, 482–489, doi:10.1016/S0141-0229(01)00523-3.
Kawai, S., Iwatsuki, M., Nakagawa, M., Inagaki, M., Hamabe, A., & Ohashi, H. (2004). An alternative β-ether cleavage pathway for a non-phenolic β-O-4 lignin model dimer catalyzed by a laccase-mediator system. Enzyme Microbial Technology, 35, 154–160, doi:10.1016/j.enzmictec.2004.03.019.
Li, K., Xu, F., & Eriksson, K.-E. L. (1999). Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Applied and Environmental Microbiology, 65, 2654–2660.
Hirai, H., Shibata, H., Kawai, S., & Nishida, T. (2006). Role of 1-hydroxybenzotriazole in oxidation by laccase from Trametes versicolor. Kinetic analysis of the laccase-1-hydroxybenzotriazole couple. FEMS Microbiology Letter, 265, 56–59, doi:10.1111/j.1574-6968.2006.00474.x.
Mester, T., & Field, J. A. (1998). Characterization of a novel manganese peroxidase-lignin peroxidase hybrid isozyme produced by Bjerkandera species strain BOS55 in the absence of manganese. Journal of Biological Chemistry, 15412–15417, doi:10.1074/jbc.273.25.15412.
Moreira, P., Duez, G., Dehareng, D., Antunes, A., Almeida-Vara, E., Frere, J. M., et al. (2005). Molecular characterization of a versatile peroxidase from a Bjerkandera strain. Journal of Biotechnology, 118, 339–352, doi:10.1016/j.jbiotec.2005.05.014.
Martinez, M., Ruiz-Duenas, F. J., Guillen, F., & Martinez, A. T. (1996). Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. European Journal of Biochemistry, 237, 424–432, doi:10.1111/j.1432-1033.1996.0424k.x.
Heinfling, A., Ruiz-Duenas, F. J., Martinez, M. J., Berbauer, M., Szewzyk, U., & Martinez, A. T. (1998). A study on reducing substrates of manganese-oxidizing peroxidases from Pleurotus eryngii and Bjerkandera adusta. FEBS Letters, 428, 141–146, doi:10.1016/S0014-5793(98)00512-2.
Wang, Y., Vazquez-Duhalt, R., & Pickard, M. A. (2003). Manganese-lignin peroxidase hybride from Bjerkandera adusta oxidizes polycyclic aromatic hydrocarbons more actively in the absence of manganese. 49, 675–682.
Camarero, S., Bockle, B., Martinez, M. J., & Martinez, A. T. (1996). Manganese-mediated lignin degradation by Pleurotus pulmonarius. Applied and Environmental Microbiology, 62, 1070–1072.
Camarero, S., Sarkar, S., Ruiz-Duenas, F. J., Martinez, M. J., & Martinez, A. T. (1999). Description of a versatile peroxidase involved in the natural degradation of lignin that has been manganese peroxidase and lignin peroxidase substrate interaction sites. Journal of Biological Chemistry, 274, 10324–10330, doi:10.1074/jbc.274.15.10324.
Kamitsuji, H., Honda, Y., Watanabe, T., & Kuwahara, M. (2005). Mn2+ is dispensable for the production of active MnP2 by Pleurotus ostreatus. Biochemistry Biophysics Research Communication, 327, 871–876, doi:10.1016/j.bbrc.2004.12.084.
Rodakiewicz-Nowak, J., Jarosz-Wilkolazka, A., & Luterek, J. (2006). Catalytic activity of versatile peroxidase from Bjerkandera fumosa in aqueous solutions of water-miscible organic solvents. Applied Catalysis A: General, 308, 56–61, doi:10.1016/j.apcata.2006.04.009.
Ruiz-Duenas, F. J., Martinez, M. J., & Martinez, A. T. (1999). Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryngii. Molecular Microbiology, 31, 223–235, doi:10.1046/j.1365-2958.1999.01164.x.
Ruiz-Duenas, F. J., Morales, M., Perez-Boada, M., Choinowski, T., Martinez, M. J., Piontek, K., et al. (2007). Manganese oxidation site in Pleurotus eryngii versatile peroxidase: A site-directed mutagenesis, kinetic, and crystallographic study. Biochemistry, 46, 66–77, doi:10.1021/bi061542h.
Perez-Boada, M., Ruiz-Duenas, F. J., Pogni, R., Basosi, R., Choinowski, T., Martinez, M., et al. (2005). Versatile peoxidase oxidation of high redox potential aromatic compounds: Site-directed mutagenesis, spectroscopic and crystallographic investigation of three long-range electron transfer pathways. Journal of Molecular Biology, 354, 385–402, doi:10.1016/j.jmb.2005.09.047.
Pongi, R., Camilla Baratto, M., Teutloff, C., Giansanti, S., Ruiz-Duenas, F. J., Choinowski, T., et al. (2006). A tryptophan neutral radical in the oxidized state of versatile peroxidase from Pleurotus eryngii. Journal of Biological Chemistry, 281, 9517–9526.
Johjima, T., Itoh, H., Kabuto, M., Tokimura, F., Nakagawa, T., Wariishi, H., et al. (1999). Direct interaction of lignin and lignin peroxidase from Phanerochaete chrysosporium. Proceedings of the National Academy of Sciences of the USA, 96, 1989–1994, doi:10.1073/pnas.96.5.1989.
Pogni, R., Camilla Baratto, M., Giansanti, S., Teutloff, C., Verdin, J., Valderrama, B., et al. (2005). Tryptophan-based radical in the catalytic mechanism of versatile peroxidase from Bjerkandera adusta. Biochemistry, 44, 4267–4274, doi:10.1021/bi047474l.
Camarero, S., Ruiz-Duenas, J., Sarkar, S., Martinez, M. J., & Martinez, A. T. (2000). The cloning of a new peroxidase found in lignocellulose cultures of Pleurotus eryngii and sequence comparison with other fungal peroxidases. FEMS Microbiology Letter, 191, 37–43, doi:10.1111/j.1574-6968.2000.tb09316.x.
Tinoco, R., Verdin, J., & Vazquez-Duhalt, R. (2007). Role of oxidizing mediators and tryptophan 172 in the decoloration of industrial dyes by the versatile peroxidases from Bjerkandera adusta. Journal of Molecular Catalysis. B, Enzymatic, 46, 1–7, doi:10.1016/j.molcatb.2007.01.006.
Mester, T., & Tien, M. (2001). Engineering of a manganese-binding site in lignin peroxidase isozyme H8 from Phanerochaete chrysosporium. Biochemical and Biophysical Research Communications, 284, 723–728, doi:10.1006/bbrc.2001.5015.
Mester, T., & Tien, M. (2000). Oxidation mechanism of ligninolytic enzymes involved in the degradation of environmental pollutants. International Biodeterioration & Biodegradation, 46, 51–59, doi:10.1016/S0964-8305(00)00071-8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reference to a company and/or products is only for purposes of information and does not imply approval of recommendation of the product to the exclusion of others that may also be suitable. All programs and services of the US Department of Agriculture are offered on a nondiscriminatory basis without regard to race, color, national origin, religion, sex, age, marital status, or handicap.
Rights and permissions
About this article
Cite this article
Wong, D.W.S. Structure and Action Mechanism of Ligninolytic Enzymes. Appl Biochem Biotechnol 157, 174–209 (2009). https://doi.org/10.1007/s12010-008-8279-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8279-z