Abstract
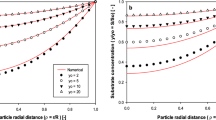
A mathematical model that describes the heterogeneous reaction–diffusion process involved in penicillin G hydrolysis in a batch reactor with immobilized penicillin G acylase is presented. The reaction system includes the bulk liquid phase containing the dissolved substrate (and products) and the solid biocatalyst phase represented by glyoxyl-agarose spherical porous particles carrying the enzyme. The equations consider reaction and diffusion components that are presented in dimensionless form. This is a complex reaction system in which both products of reaction and the substrate itself are inhibitors. The simulation of a batch reactor performance with immobilized penicillin G acylase is presented and discussed for the internal diffusional restrictions impact on effectiveness and productivity. Increasing internal diffusional restrictions, through increasing catalyst particle size and enzyme loading, causes impaired catalyst efficiency expressed in a reduction of effectiveness factor and specific productivity. High penicillin G initial concentrations decrease the impact of internal diffusional restrictions by increasing the mass transfer towards porous catalyst until product inhibition becomes significant over approximately 50 mM of initial penicillin G, where a drop in conversion rate and a maximum in specific productivity are then obtained. Results highlight the relevance of considering internal diffusional restrictions, reactor performance, and productivity analysis for proper catalyst and reactor design.







Similar content being viewed by others
Abbreviations
- A :
-
Total catalyst surface area
- D e :
-
Penicillin G effective diffusion coefficient
- D e1 :
-
PAA-effective diffusion coefficient
- D e2 :
-
APA-effective diffusion coefficient
- E :
-
Catalyst enzyme concentration
- E R :
-
Reactor enzyme concentration
- k :
-
Reaction rate constant
- K :
-
Michaelis constant for penicillin G
- K 1 :
-
Competitive product inhibition constant for PAA
- K 2 :
-
Non-competitive product inhibition constant for APA
- K S :
-
Uncompetitive inhibition constant for penicillin G
- p :
-
Dimensionless PAA concentration
- P 1 :
-
PAA concentration
- P 1b :
-
Bulk PAA concentration
- P 2 :
-
APA concentration
- P 2b :
-
Bulk APA concentration
- q :
-
Dimensionless APA concentration
- Q p :
-
Volumetric productivity
- Q pe :
-
Specific productivity
- r :
-
Variable radius inside the catalyst particle
- R :
-
Catalyst particle radius
- S :
-
Substrate concentration inside catalyst
- s :
-
Dimensionless substrate concentration
- S b :
-
Bulk substrate concentration
- S b0 :
-
Initial bulk substrate concentration
- t :
-
Reaction time
- T :
-
Characteristic time (R 2/D e)
- v :
-
Reaction rate
- V b :
-
Bulk volume of liquid phase
- V c :
-
Total volume of catalyst particles in reactor
- γ :
-
Liquid to catalyst volume ratio: V b /A ∙ R or V b /3 ∙ V c
- η :
-
Effectiveness factor
- η′:
-
Overall effectiveness factor
- ρ :
-
Dimensionless radius (r/R)
- σ :
-
Dimensionless reaction rate
- τ :
-
Dimensionless reaction time
- Φ:
-
Thiele modulus for Penicillin G
- Φ1 :
-
Thiele modulus for PAA
- Φ2 :
-
Thiele modulus for APA
References
Mateo, C., Abián, O., Bernedo, M., Cuenca, E., Fuentes, M., Fernández-Lorente, G., et al. (2005). Enzyme and Microbial Technology, 37, 456–462.
Mateo, C., Palomo, J. M., Fuentes, M., Betancor, L., Grazu, V., López-Gallego, F., et al. (2006). Enzyme and Microbial Technology, 39, 274–280.
Illanes, A., & Wilson, L. (2003). Critical Reviews in Biotechnology, 23, 61–93.
Shuler, M., Aris, R., & Tsuchiya, H. (1972). Journal of Theoretical Biology, 35, 67–76.
Engasser, J., & Horvath, C. (1973). Journal of Theoretical Biology, 42, 137–155.
Al-Muftah, A., & Abu-Reesh, I. (2005). Biochemical Engineering Journal, 23, 139–153.
Illanes, A. (2008). Enzyme biocatalysis: Principles and applications. London: Springer.
Li, X., Chen, X., & Chen, N. (2004). Biochemical Engineering Journal, 17, 65–69.
Kheirolomoom, A., Khorasheh, F., & Fazelinia, H. (2002). Journal of Bioscience and Bioengineering, 93, 125–129.
Van Roon, J. L., Arntz, M. M. H. D., Kallenberg, A. I., x Paasman, A. I., Tramper, J., Schroën, C. G. H. P., et al. (2006). Applied Microbiology and Biotechnology, 72, 263–278.
Illanes, A., & Wilson, L. (2006). Chimica Oggi/Chemicals Today, 24, 27–30.
Kheirolomoom, A., Ardjmand, M., Fazelinia, H., & Zakeri, A. (2001). Process Biochemistry, 36, 1095–1101.
Balasingham, K., Warburton, D., Dunill, P., & Lilly, M. D. (1972). Biochimica et Biophysica Acta Enzymology, 276, 250–256.
Warburton, D., Dunnill, P., & Lilly, M. (1973). Biotechnology and Bioengineering, 15, 13–25.
Lee, S. B., & Ryu, D. (1982). Enzyme and Microbial Technology, 4, 35–38.
Valencia, P., Flores, S., Wilson, L., & Illanes, A. (2010). Biochemical Engineering Journal, 49, 256–263.
Guisán, J. M., Alvaro, G., Rosell, C., & Fernandez-Lafuente, R. (1994). Biotechnology and Applied Biochemistry, 20, 357–369.
Ospina, S., López-Mungia, A., González, R., & Quintero, R. (1992). Journal of Chemical Technology and Biotechnology, 53, 205–213.
Shewale, J., & Sivaraman, H. (1989). Process Biochemistry, 24, 146–154.
Acknowledgements
Work funded by Chilean Fondecyt Project 1080122.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Simulation was done by solving Eqs. 22–24 and 32–34. Dimensionless equations were discretized using Crank–Nicolson method to achieve stability during solution processing in computer programs. The number of equations depends on N obtaining N + 1 equations for each substance. In this study, N = 20 was used obtaining a system of 63 equations. Algebraic form of discretized equations are as follows:For j = 0:
For j = 1,…, N −1:
For j = N:
Rights and permissions
About this article
Cite this article
Valencia, P., Flores, S., Wilson, L. et al. Effect of Internal Diffusional Restrictions on the Hydrolysis of Penicillin G: Reactor Performance and Specific Productivity of 6-APA with Immobilized Penicillin Acylase. Appl Biochem Biotechnol 165, 426–441 (2011). https://doi.org/10.1007/s12010-011-9262-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9262-7