Abstract
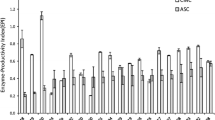
The development of new cost-effective bioprocesses for the production of cellulolytic enzymes is needed in order to ensure that the conversion of biomass becomes economically viable. The aim of this study was to determine whether a novel sequential solid-state and submerged fermentation method (SF) could be validated for different strains of the Trichoderma genus. Cultivation of the Trichoderma reesei Rut-C30 reference strain under SF using sugarcane bagasse as substrate was shown to be favorable for endoglucanase (EGase) production, resulting in up to 4.2-fold improvement compared with conventional submerged fermentation. Characterization of the enzymes in terms of the optimum pH and temperature for EGase activity and comparison of the hydrolysis profiles obtained using a synthetic substrate did not reveal any qualitative differences among the different cultivation conditions investigated. However, the thermostability of the EGase was influenced by the type of carbon source and cultivation system. All three strains of Trichoderma tested (T. reesei Rut-C30, Trichoderma harzianum, and Trichoderma sp INPA 666) achieved higher enzymatic productivity when cultivated under SF, hence validating the proposed SF method for use with different Trichoderma strains. The results suggest that this bioprocess configuration is a very promising development for the cellulosic biofuels industry.




Similar content being viewed by others
References
Klein-Marcuschamer, D., Oleskowicz-Popiel, P., Simmons, B. A., & Blanch, H. W. (2012). The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnology and Bioengineering, 109, 1083–1087.
Singhania, R. R., Sukumaran, R. K., Patel, A. K., Larroche, C., & Pandey, A. (2010). Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme and Microbial Technology, 46, 541–549.
Delabona, P., Farinas, C., da Silva, M., Azzoni, S., & Pradella, J. (2012). Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugar cane bagasse for on-site cellulase production. Bioresource Technology, 107, 517–521.
Sorensen, A., Teller, P. J., Lubeck, P. S., & Ahring, B. K. (2011). Onsite enzyme production during bioethanol production from biomass: screening for suitable fungal strains. Applied Biochemistry and Biotechnology, 164, 1058–1070.
Kovacs, K., Macrelli, S., Szakacs, G., & Zacchi, G. (2009). Enzymatic hydrolysis of steam-pretreated lignocellulosic materials with Trichoderma atroviride enzymes produced in-house. Biotechnology for Biofuels, 2, 11.
Rana, V., Eckard, A. D., Teller, P., & Ahring, B. K. (2014). On-site enzymes produced from Trichoderma reesei RUT-C30 and Aspergillus saccharolyticus for hydrolysis of wet exploded corn stover and loblolly pine. Bioresource Technology, 154, 282–289.
Furlan, F. F., Tonon, R., Pinto, F., Costa, C. B. B., Cruz, A. J. G., Giordano, R. L. C., & Giordano, R. C. (2013). Bioelectricity versus bioethanol from sugarcane bagasse: is it worth being flexible? Biotechnology for Biofuels, 6, 12.
Gusakov, A. V. (2011). Alternatives to Trichoderma reesei in biofuel production. Trends in Biotechnology, 29, 419–425.
Hasunuma, T., Okazaki, F., Okai, N., Hara, K. Y., Ishii, J., & Kondo, A. (2013). A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology. Bioresource Technology, 135, 513–522.
Jourdier, E., Cohen. C., Poughon, L., Larroche, C., Monot, F., Ben Chaabane, F. (2013). Cellulase activity mapping of Trichoderma reesei cultivated in sugar mixtures under fed-batch conditions. Biotechnology for Biofuels, 6.
Viikari, L., Alapuranen, M., Puranen, T., Vehmaanpera, J., & Siika-Aho, M. (2007). Thermostable enzymes in lignocellulose hydrolysis. In L. Olsson (Ed.), Biofuels. Advances in biochemical engineering–Biotechnology (Vol. 108, pp. 121–145). Berlin: Springer-Verlag Berlin.
Florencio, C., Couri, S., Farinas, C.S. (2012). Correlation between agar plate screening and solid-state fermentation for the prediction of cellulase production by Trichoderma strains. In Enzyme Research. pp. 7:7.
Barrios-Gonzalez, J. (2012). Solid-state fermentation: physiology of solid medium, its molecular basis and applications. Process Biochemistry, 47, 175–185.
Farinas, C., Vitcosque, G., Fonseca, R., Neto, V., & Couri, S. (2011). Modeling the effects of solid state fermentation operating conditions on endoglucanase production using an instrumented bioreactor. Industrial Crops and Products, 34, 1186–1192.
Thomas, L., Larroche, C., & Pandey, A. (2013). Current developments in solid-state fermentation. Biochemical Engineering Journal, 81, 146–161.
Cunha, F. M., Esperanca, M. N., Zangirolami, T. C., Badino, A. C., & Farinas, C. S. (2012). Sequential solid-state and submerged cultivation of Aspergillus niger on sugarcane bagasse for the production of cellulase. Bioresource Technology, 112, 270–274.
Delabona, P., Pirota, R., Codima, C., Tremacoldi, C., Rodrigues, A., & Farinas, C. (2012). Using Amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biomass & Bioenergy, 37, 243–250.
Mandels, M., & Sternberg, D. (1976). Recent advances in cellulase technology. Journal of Fermentation Technology, 54, 267–286.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure & Applied Chemistry, 59, 257–268.
Bailey, M. J., & Poutanen, K. (1989). Production of xylanolytic enzymes by strains of Aspergillus. Applied Microbiology and Biotechnology, 30, 5–10.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Bradford, M. M. (1976). Rapid and sensitive method for quantification of microgram quantities of protein utilizing principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Sadana, A., & Henley, J. P. (1987). Single-step unimolecular non-1st-order enzyme deactivation kinetics. Biotechnology and Bioengineering, 30, 717–723.
Ahamed, A., & Vermette, P. (2009). Effect of culture medium composition on Trichoderma reesei's morphology and cellulase production. Bioresource Technology, 100, 5979–5987.
Domingues, F. C., Queiroz, J. A., Cabral, J. M. S., & Fonseca, L. P. (2000). The influence of culture conditions on mycelial structure and cellulase production by Trichoderma reesei Rut C-30. Enzyme and Microbial Technology, 26, 394–401.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., & Ladisch, M. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96, 673–686.
Rodriguez-Zuniga, U. F., Neto, V. B., Couri, S., Crestana, S., & Farinas, C. S. (2014). Use of spectroscopic and imaging techniques to evaluate pretreated sugarcane bagasse as a substrate for cellulase production under solid-state fermentation. Applied Biochemistry and Biotechnology, 172, 2348–2362.
Farinas, C., Loyo, M., Baraldo, A., Tardioli, P., Neto, V., & Couri, S. (2010). Finding stable cellulase and xylanase evaluation of the synergistic effect of pH and temperature. New Biotechnology, 27, 810–815.
Xing, S., Li, G. L., Sun, X. L., Ma, S., Chen, G. J., Wang, L. S., & Gao, P. J. (2013). Dynamic changes in xylanases and beta-1,4-endoglucanases secreted by Aspergillus niger An-76 in response to hydrolysates of lignocellulose polysaccharide. Applied Biochemistry and Biotechnology, 171, 832–846.
Samanta, S., Basu, A., Halder, U. C., & Sen, S. K. (2012). Characterization of Trichoderma reesei endoglucanase II expressed heterologously in Pichia pastoris for better biofinishing and biostoning. Journal of Microbiology, 50, 518–525.
de Castro, A. M., Pedro, K., da Cruz, J. C., Ferreira, M. C., Leite, S. G. F., & Pereira, N. (2010). Trichoderma harzianum IOC-4038: a promising strain for the production of a cellulolytic complex with significant beta-glucosidase activity from sugarcane bagasse cellulignin. Applied Biochemistry and Biotechnology, 162, 2111–2122.
Ximenes, E., Kim, Y., Mosier, N., Dien, B., & Ladisch, M. (2010). Inhibition of cellulases by phenols. Enzyme and Microbial Technology, 46, 170–176.
Ximenes, E., Kim, Y., Mosier, N., Dien, B., & Ladisch, M. (2011). Deactivation of cellulases by phenols. Enzyme and Microbial Technology, 48, 54–60.
Saqib, A. A. N., Hassan, M., Khan, N. F., & Baig, S. (2010). Thermostability of crude endoglucanase from Aspergillus fumigatus grown under solid state fermentation (SSF) and submerged fermentation (SmF). Process Biochemistry, 45, 641–646.
Kim, Y., Mosier, N. S., & Ladisch, M. R. (2009). Enzymatic digestion of liquid hot water pretreated hybrid poplar. Biotechnology Progress, 25, 340–348.
Cao, G. L., Ximenes, E., Nichols, N. N., Zhang, L. Y., & Ladisch, M. (2013). Biological abatement of cellulase inhibitors. Bioresource Technology, 146, 604–610.
Grigorevski-Lima, A. L., Quadros de Oliveira, M. M., do Nascimento, R. P., da Silva Bon, E. P., & Rodrigues Coelho, R. R. (2013). Production and partial characterization of cellulases and xylanases from Trichoderma atroviride 676 using lignocellulosic residual biomass. Applied Biochemistry and Biotechnology, 169, 1373–1385.
Acknowledgments
The authors thank the Brazilian agencies Fapesp, Capes, and CNPq for financial support, and the staff of Embrapa Instrumentation for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Florencio, C., Cunha, F.M., Badino, A.C. et al. Validation of a Novel Sequential Cultivation Method for the Production of Enzymatic Cocktails from Trichoderma Strains. Appl Biochem Biotechnol 175, 1389–1402 (2015). https://doi.org/10.1007/s12010-014-1357-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1357-5