Abstract
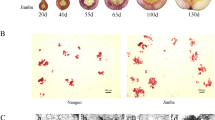
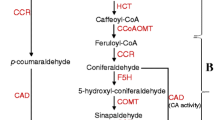
Lignin is a versatile plant metabolite challenging high-end industrial applications of several plant products including jute. Application of developmental mutant in regulation of lignification in jute may open up door for much awaited jute based diversified products. In the present study, a novel dark jute (Corchorus olitorius L.) mutant with low lignin (7.23%) in phloem fibre being compared to wild-type JRO 204 (13.7%) was identified and characterised. Unique morphological features including undulated stem, petiole and leaf vein distinguished the mutant in gamma ray irradiated mutant population. Histological and biochemical analysis revealed reduced lignification of phloem fibre cells of the plant. RT-PCR analysis demonstrated temporal transcriptional regulation of CCoAMT1 gene in the mutant. The mutant was found an extremely useful model to study phloem fibre developmental biology in the crop besides acting as a donor genetic stock for low lignin containing jute fibre in dark jute improvement programme.




Similar content being viewed by others
References
Karmakar, P. G., Hazra, S. K., Sinha, M. K., & Chaudhury, S. K. (2008). Breeding for quantitative traits and varietal development in jute and allied fibre crops. In P. G. Karmakar, S. K. Hazra, T. Ramasubramanian, R. K. Mandal, M. K. Sinha, & H. S. Sen (Eds.), Jute and allied fibre updates: production and technology (pp. 18–37). Barrackpore: Central Research Institute for Jute and Allied Fibres.
Summerscales, J., Dissanayake, N. P. J., Virk, A. S., & Hall, W. (2010). A review of bast fibres and their composites. Part 1—fibres as reinforcements. Composites Part A Applied Science and Manufacturing, 41, 1329–1335. doi:10.1016/j.compositesa.2010.06.001.
Rio, J. C., Del, R. J., Marques, G., Li, J., Gellerstedt, G., Barbero, J. J., Martinez, A. T., & Gutierrez, A. (2009). Structural characterization of the lignin from jute (Corchorus capsularis) fibers. Journal of Agricultural and Food Chemistry, 57, 10271–10281. doi:10.1021/jf900815x.
Kundu, B. C. (1944). Anatomy of jute stem with special reference to cambial activity and distribution of fibres in relation to leaf-trace system. J Royal Asiatic Soc Bengal (Sci)., 10, 27–52.
Kundu, B. C., Basak, K. C., & Sarkar, P. B. (1959). Jute in India. Calcutta: The Indian Central Jute Committee.
Maiti, R. K. (1997). World fibre crops. New Delhi: Oxford and IBH Publishing Company.
Hazra, S. K., & Karmakar, P. G. (2008). Anatomical parameters of bast fibres for yield and quality improvement. In P. G. Karmakar, S. K. Hazra, T. Ramasubramanian, R. K. Mandal, M. K. Sinha, & H. S. Sen (Eds.), Jute and allied fibre updates (pp. 38–45). Kolkata: Central Research Institute for Jute and Allied Fibres.
Day, A., Ruel, K., Neutelings, G., Cronier, D., David, H., Hawkins, S., & Chabbert, B. (2005). Lignification in the flax stem: evidence for an unusual lignin in bast fibres. Planta, 222, 234–245. doi:10.1007/s00425-005-1537-1.
Rowell, R. M., & Stout, H. P. (2007). Jute and kenaf. In M. Lewin (Ed.), Handbook of fibre chemistry (3rd ed., pp. 405–452). Boca Raton: CRC Press.
Maiti, R. K., & Mitra, G. C. (1972). Cambial activity in relation to the production of fibre bundles at different phases of growth in white jute. Bull Bot Soc Bengal, 26, 79–85.
Mitra, G. C. (1984). Genetic control of secondary phloic fibres in jute (Corchorus capsularis L.) Genetica, 63, 9–11.
Palit, P., & Meshram, J. H. (2004). Physiological characterization of a phenotypically distinct jute (Corchorus olitorius) genotype. Plant Genet. Resour., 2, 175–180.
Sengupta, G., & Palit, P. (2004). Characterization of a lignified secondary phloem fibre-deficient mutant of jute (Corchorus capsularis). Annals of Botany, 93, 211–220. doi:10.1093/aob/mch029.
Meshram, J. H., & Palit, P. (2013). On the role of cell wall lignin in determining the fineness of jute fibre. Acta Physiologiae Plantarum, 35, 1565–1578.
Campbell, M. M., & Sederoff, R. R. (1996). Variation in lignin content and composition. Mechanisms of control and implications for the genetic improvement of plants. Plant Physiology, 110, 3–13.
Wagner, G. P., & Lynch, V. J. (2008). The gene regulatory logic of transcription factor evolution. Trends in Ecology & Evolution, 23, 377–385. doi:10.1016/j.tree.2008.03.006.
Ralph, J., Hatfield, R. D., Piquemal, J., Yahiaoui, N., & Pean, M. (1998). NMR characterization of altered lignins extracted from tobacco plants down regulated for lignification enzymes cinnamyl-alcohol dehydrogenase and cinnamoyl-CoA reductase. Proceedings of the National Academy of Sciences of the United States of America, 95, 12803–12808. doi:10.1073/pnas.95.22.12803.
Ralph, J., Kim, H., Peng, J., & Lu, F. (1999). Arylpropane-1,3-diols in lignins from normal and CAD-deficient pines. Organic Letters, 1, 323–326. doi:10.1021/ol9906559.
Ralph, J., Lapierre, C., Lu, F., Marita, J. M., & Pilate, G. (2001). NMR evidence for benzodioxane structures resulting from incorporation of 5-hydroxyconiferyl alcohol into lignins of O-methyltransferase-deficient plants. Journal of Agricultural and Food Chemistry, 49, 86–91. doi:10.1021/jf001042.
Provan, G. J., Scobbie, L., & Chesson, A. (1997). Characterisation of lignin from CAD and OMT deficient bm mutants of maize. Journal of the Science of Food and Agriculture, 73, 133–142. doi:10.1002/(SICI)1097-0010(199702)73:2<133::AID-JSFA696>3.0.CO;2-Q.
Piquemal, J., Chamayou, S., Nadaud, I., Beckert, M., & Barriere, Y. (2002). Down-regulation of caffeic acid omethyltransferase in maize revisited using a transgenic approach. Plant Physiology, 130, 1675–1685. doi:10.1104/pp.012237.
Jones, L., Ennos, A. R., & Turner, S. R. (2001). Cloning and characterization of irregular xylem4 (irx4): a severely lignin-deficient mutant of Arabidopsis. The Plant Journal, 26, 205–216. doi:10.1046/j.1365-313x.2001.01021.x.
Leplé, J. C., Dauwe, R., Morreel, K., Storme, V., Lapierre, C., Pollet, B., Naumann, A., & Leroux, O. (2012). Collenchyma: a versatile mechanical tissue with dynamic cell walls. Annals of Botany, 110, 1083–1098. doi:10.1093/aob/mcs186.
Zhou, R., Jackson, L., Shadle, G., Nakashima, J., Temple, S., Chen, F., & Dixon, R. A. (2010). Distinct cinnamoyl CoA reductases involved in parallel routes to lignin in Medicago truncatula. Proceedings of the National Academy of Sciences of the United States of America, 107, 17803–17808. doi:10.1073/pnas.1012900107.
Rogers, L. A., & Campbell, M. M. (2004). The genetic control of lignin deposition during plant growth and development. The New Phytologist, 164, 17–30. doi:10.1111/j.1469-8137.2004.01143.x.
Bandopadhyay, S. B., & Mukhopadhyay, S. K. (1964). Assessment of jute fibre bundle strength. Jute Bull., 27, 193–199.
Sinha, N. G., & Bandopadhyay, S. B. (1968). An air-flow method for the determination of the fibre fineness of jute and mesta. Journal of the Textile Institute, 59, 148–156.
Kundu, A., Sarkar, D., Mandal, N. A., Sinha, M. K., & Mahapatra, B. S. (2012). A secondary phloic (bast) fibre-shy (bfs) mutant of dark jute (Corchorus olitorius L.) develops lignified fibre cells but is defective in cambial activity. Plant Growth Regulation, 67(1), 45–55. doi:10.1007/s10725-012-9660-z.
Sengupta, A. B., Mazumdar, S. K., & Macmillan, N. G. (1958). Isolation of jute holocellulose by the action of sodium chlorite. Indian J Appl Chem., 21(10), 105.
Dashek, W. V. (1997). Methods in plant biochemistry and molecular biology. Boca Raton: CRC Press.
Inc, S. P. S. S. Released 2007. SPSS for Windows, version 16.0. Chicago: SPSS Inc..
Kundu, A., Sarkar, D., Bhattacharjee, A., Topdar, N., Sinha, M. K., & Mahapatra, B. S. (2011). A simple ethanol wash of the tissue homogenates recovers high-quality genomic DNA from Corchorus species characterized by highly acidic and proteinaceous mucilages. Electronic Journal of Biotechnology, 14(1). doi:10.2225/vol14-issue1-fulltext-4.
Choudhary, S. B., Kumar, M., Chowdhury, I., Singh, R. K., Pandey, S. P., Sharma, H. K., & Karmakar, P. G. (2016). An efficient and cost effective method of RNA extraction from mucilage, phenol and secondary metabolite rich bark tissue of tossa jute (C. olitorius L.) actively developing phloem fiber. 3Biotech, 6, 100. doi:10.1007/s13205-016-0415-9.
Zhang, G., Zhang, Y., Xu, J., Niu, X., Qi, J., Tao, A., Zhang, L., Fang, P., Lin, L., & Hui, J. S. (2014). The CCoAOMT1 gene from jute (Corchorus capsularis L.) is involved in lignin biosynthesis in Arabidopsis thaliana. Gene, 546, 398–402.
Chakraborty, A., Sarkar, D., Satya, P., Karmakar, P. G., & Singh, N. K. (2015). Pathways associated with lignin biosynthesis in lignomaniac jute fibres. Molecular Genetics and Genomics, 290, 1523–1542. doi:10.1007/s00438-015-1013-y.
Lewis, N. G., & Yamamoto, E. (1990). Lignin: Occurrence, biogenesis and biodegradation. Annual Review of Plant Physiology and Plant Molecular Biology, 41, 455–496. doi:10.1146/annurev.pp.41.060190.002323.
Kokubo, A., Kuraishi, S., & Sakurai, N. (1989). Culm strength of barley: correlation among maximum bending stress, cell wall dimensions, and cellulose content. Plant Physiology, 91, 876–882.
Kokubo, A., Sakurai, N., Kuraishi, S., & Takabe, K. (1991). Culm brittleness of barley (Hordeum vulgare L.) mutants is caused by smaller number of cellulose molecules in cell wall. Plant Physiology, 97, 509–514. doi:10.1104/pp.91.3.876.
Qian, Q., Li, Y. H., Zeng, D., Teng, S., Wang, Z., Li, X., Dong, Z., Dai, N., Sun, L., & Li, J. (2001). Isolation and genetic characterization of a fragile plant mutant rice (Oryza sativa L.) Chinese Science Bulletin, 46, 2082–2085. doi:10.1007/BF02901137.
Snegireva, A., Tatyana, C., Marina, A., Simcha, L., & Tatyana, G. (2015). Intrusive growth of primary and secondary phloem fibres in hemp stem determines fibre bundle formation and structure. AoB PLANTS. doi:10.1093/aobpla/plv061.
Meshitsuka, G., & Nakano, J. (1979). Studies on the mechanism of lignin color reaction (XIII): Maule color reaction (9). Mokuzai Gakkaishi, 25, 588–594.
Zhao, H. Y. (2005). Isolation and functional characterisation of cinamate-4 hydroxylase promoter from Populous tomentosa L. Plant Science, 168, 1157–1162. doi:10.1016/j.plantsci.2004.12.022.
Mele, G., Orie, N., Sato, Y., & Hake, S. (2003). The knotted-1 like homeobox gene BREVIPEDICELLUS regulates cell differentiation by modulating metabolic pathways. Genes & Development, 17, 2088–2093. doi:10.1101/gad.1120003.
Liljegren, S. J., Ditta, G. S., Eshed, H. Y., Savidge, B., Bowman, J. L., & Yanofsky, M. F. (2000). Shatter proof MADS-box genes control seed dispersal in Arabidopsis. Nature, 404(6779), 766–770. doi:10.1038/35008089.
Zhong, R., Burk, D. H., Morrison, W. H., & Ye, Z. H. (2002). A kinesin like protein is essential for oriented deposition of cellulose microfibrils and cell wall strength. Plant Cell, 14, 3101–3117. doi:10.1105/tpc.005801.
Bell-Lelong, D. A., Cusumano, J. C., Meyer, K., & Chapple, C. (1997). Cinnamate-4-hydroxylase expression in Arabidopsis (regulation in response to development and the environment). Plant Physiology, 113(3), 729–738. doi:10.1104/pp.113.3.729.
Cabane, M., Pireaux, J. C., Leger, E., Weber, E., Dizengremel, P., Pollet, B., & Lapierre, C. (2004). Condensed lignins are synthesized in poplar leaves exposed to ozone. Plant Physiology, 134, 586–594. doi:10.1104/pp.103.031765.
Acknowledgements
The authors acknowledge the financial assistance for the work received in the form of research grant (35/14/01/2014-BRNS) from BRNS, Department of Atomic Energy, Govt. of India. Valuable suggestions given by Dr. D. Sarkar, Pr. Scientist, ICAR-CRIJAF, for data presentation and manuscript writing are deeply acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Supplementary file 1
Phenotype of wild type JRO 204 plant, stem, leaf, root (a-d) and llpf mutant plant, stem, leaf, root (e-h). (GIF 518 kb)
Supplementary file 2
PCR analysis for CCoAMT1 gene using genomic DNA. 100 bp DNA ladder (a), wild type JRO 204 (b), llpf mutant (c). (GIF 763 kb)
Supplementary file 3
RT-PCR analysis for CCoAMT1 gene. 100 bp DNA ladder (a), wild-type JRO 204 at 20 DAS (b), 45 DAS (d), 70 DAS (f) and llpf mutant at 20 DAS (c), 45 DAS (e), 70 DAS (g). (GIF 5993 kb)
Rights and permissions
About this article
Cite this article
Choudhary, S.B., Chowdhury, I., Singh, R.K. et al. Morphological, Histobiochemical and Molecular Characterisation of Low Lignin Phloem Fibre (llpf) Mutant of Dark Jute (Corchorus olitorius L.). Appl Biochem Biotechnol 183, 980–992 (2017). https://doi.org/10.1007/s12010-017-2477-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2477-5