Abstract
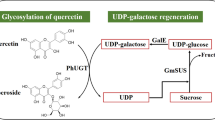
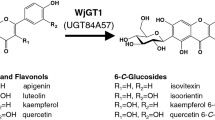
Isoorientin and isovitexin, kinds of flavone C-glycosides, exhibit a number of biological properties. In this work, The C-glucosyltransferase (Gt6CGT) gene from Gentiana triflora was cloned and expressed in Escherichia coli BL21(DE3). The optimal activity of Gt6CGT was at pH 7.5 and 50° C. The enzyme was stable over pH range of 6.5–9.0, and had a 1-h half-life at 50° C. The Vmax for luteolin and apigenin was 21.1 nmol/min/mg and 31.7 nmol/min/mg, while the Km was 0.21 mM and 0.22 mM, respectively. Then, we developed an environmentally safe and efficient method for isoorientin and isovitexin production using the coupled catalysis of Gt6CGT and Glycine max sucrose synthase (GmSUS). By optimizing coupled reaction conditions, the titer of isoorientin and isovitexin reached 3820 mg/L with a corresponding molar conversion of 94.7% and 3772 mg/L with a corresponding molar conversion of 97.1%, respectively. The maximum number of UDP-glucose regeneration cycles (RCmax) reached 28.4 for isoorientin and 29.1 for isovitexin. The coupled catalysis reported herein represents a promising method to meet industrial requirements for large-scale isoorientin and isovitexin production in the future.

Graphical Abstract








Similar content being viewed by others
References
Dixon, R. A., & Paiva, N. L. (1995). Stress-induced phenylpropanoid metabolism. Plant Cell, 7(7), 1085–1097.
Yonekura-Sakakibara, K., Tohge, T., Niida, R., & Saito, K. (2007). Identification of a flavonol 7-O-rhamnosyltransferase gene determining flavonoid pattern in Arabidopsis by transcriptome coexpression analysis and reverse genetics. The Journal of Biological Chemistry, 282(20), 14932–14941.
Nijveldt, R. J., van Nood, E., van Hoorn, D. E., Boelens, P. G., van Norren, K., & van Leeuwen, P. A. (2001). Flavonoids: a review of probable mechanisms of action and potential applications. The American Journal of Clinical Nutrition, 74(4), 418–425.
Wu, X., Chu, J., Wu, B., Zhang, S., & He, B. (2013). An efficient novel glycosylation of flavonoid by beta-fructosidase resistant to hydrophilic organic solvents. Bioresource Technology, 129, 659–662.
An, D. G., Yang, S. M., Kim, B. G., & Ahn, J. H. (2016). Biosynthesis of two quercetin O-diglycosides in Escherichia coli. Journal of Industrial Microbiology & Biotechnology, 43(6), 841–849.
Durr, C., Hoffmeister, D., Wohlert, S. E., Ichinose, K., Weber, M., Von Mulert, U., Thorson, J. S., & Bechthold, A. (2004). The glycosyltransferase UrdGT2 catalyzes both C- and O-glycosidic sugar transfers. Angewandte Chemie International Edition, 43(22), 2962–2965.
Pacifico, S., Scognamiglio, M., D’Abrosca, B., Piccolella, S., Tsafantakis, N., Gallicchio, M., Ricci, A., & Fiorentino, A. (2010). Spectroscopic characterization and antiproliferative activity on HepG2 human hepatoblastoma cells of flavonoid C-glycosides from Petrorhagia velutina. Journal of Natural Products, 73(12), 1973–1978.
Yuan, L., Wang, J., Xiao, H., Wu, W., Wang, Y., & Liu, X. (2013). MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food and Chemical Toxicology, 53(3), 62–68.
Yuan, L., Wei, S., Wang, J., & Liu, X. (2014). Isoorientin induces apoptosis and autophagy simultaneously by reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38 signaling pathways in HepG2 cancer cells. Journal of Agricultural and Food Chemistry, 62(23), 5390–5400.
Zhang, Y., Jiao, J., Liu, C., Wu, X., & Zhang, Y. (2007). Isolation and purification of four flavone C -glycosides from antioxidant of bamboo leaves by macroporous resin column chromatography and preparative high-performance liquid chromatography. Food Chemistry, 107(3), 1326–1336.
Hu, C., Zhang, Y., & Kitts, D. D. (2000). Evaluation of antioxidant and prooxidant activities of bamboo Phyllostachys nigra var. Henonis leaf extract in vitro. Journal of Agricultural and Food Chemistry, 48(8), 3170–3176.
Yuan, L., Han, X., Li, W., Ren, D., & Yang, X. (2016). Isoorientin prevents hyperlipidemia and liver injury by regulating lipid metabolism, antioxidant capability, and inflammatory cytokine release in high-fructose-fed mice. Journal of Agricultural and Food Chemistry, 64(13), 2682–2689.
Luan, G., Wang, Y., Wang, Z., Zhou, W., Hu, N., Li, G., & Wang, H. (2018). Flavonoid glycosides from fenugreek seeds regulate glycolipid metabolism by improving mitochondrial function in 3T3-L1 adipocytes in vitro. Journal of Agricultural and Food Chemistry, 66(12), 3169–3178.
Yuan, L., Li, X., He, S., Gao, C., Wang, C., & Shao, Y. (2018). Effects of natural flavonoid isoorientin on growth performance and gut microbiota of mice. Journal of Agricultural and Food Chemistry, 66(37), 9777–9784.
Zhang, Y., Bao, B., Lu, B., Ren, Y., & Tie, X. (2005). Determination of flavone C-glucosides in antioxidant of bamboo leaves (AOB) fortified foods by reversed-phase high-performance liquid chromatography with ultraviolet diode array detection. Journal of Chromatography A, 1065(2), 177–185.
Kim, Y. C., Jun, M., Jeong, W. S., & Chung, S. K. (2010). Antioxidant properties of flavone C-glycosides from Atractylodes japonica leaves in human low-density lipoprotein oxidation. Journal of Food Science, 70(9), S575–S580.
De Bruyn, F., Van Brempt, M., Maertens, J., Van Bellegem, W., Duchi, D., & De Mey, M. (2015). Metabolic engineering of Escherichia coli into a versatile glycosylation platform: production of bio-active quercetin glycosides. Microbial Cell Factories, 14(1), 138.
Kim, H. J., Kim, B. G., & Ahn, J. H. (2013). Regioselective synthesis of flavonoid bisglycosides using Escherichia coli harboring two glycosyltransferases. Applied Microbiology and Biotechnology, 97(12), 5275–5282.
Pei, J., Dong, P., Wu, T., Zhao, L., Fang, X., Cao, F., Tang, F., & Yue, Y. (2016). Metabolic engineering of Escherichia coli for astragalin biosynthesis. Journal of Agricultural and Food Chemistry, 64(42), 7966–7972.
Brazier-Hicks, M., & Edwards, R. (2013). Metabolic engineering of the flavone-C-glycoside pathway using polyprotein technology. Metabolic Engineering, 16, 11–20.
Sasaki, N., Nishizaki, Y., Yamada, E., Tatsuzawa, F., Nakatsuka, T., Takahashi, H., & Nishihara, M. (2015). Identification of the glucosyltransferase that mediates direct flavone C-glucosylation in Gentiana triflora. FEBS Letters, 589(1), 182–187.
Shrestha, A., Pandey, R. P., Dhakal, D., Parajuli, P., & Sohng, J. K. (2018). Biosynthesis of flavone C-glucosides in engineered Escherichia coli. Applied Microbiology and Biotechnology, 102(3), 1251–1267.
Pei, J., Sun, Q., Zhao, L., Shi, H., Tang, F., & Cao, F. (2019). Efficient biotransformation of luteolin to isoorientin through adjusting induction strategy, controlling acetic acid and increasing UDP-glucose supply in Escherichia coli. Journal of Agricultural and Food Chemistry, 67(1), 331–340.
Pei, J., Chen, A., Zhao, L., Cao, F., Ding, G., & Xiao, W. (2017). One-pot synthesis of hyperoside by a three-enzyme cascade using a UDP-galactose regeneration system. Journal of Agricultural and Food Chemistry, 65(29), 6042–6048.
Bungaruang, L., Gutmann, A., & Nidetzky, B. (2013). Leloir glycosyltransferases and natural product glycosylation: biocatalytic synthesis of the C-glucoside nothofagin, a major antioxidant of Redbush Herbal Tea. Advanced Synthesis & Catalysis, 355(14-15), 2757–2763.
Gutmann, A., Bungaruang, L., Weber, H., Leypold, M., Breinbauer, R., & Nidetzky, B. (2014). Towards the synthesis of glycosylated dihydrochalcone natural products using glycosyltransferase-catalysed cascade reactions. Green Chemistry, 16(9), 4417–4425.
Pei, J., Chen, A., Sun, Q., Zhao, L., Cao, F., & Tang, F. (2018). Construction of a novel UDP-rhamnose regeneration system by a two-enzyme reaction system and application in glycosylation of flavonoid. Biochemical Engineering Journal, 139, 33–42.
Qian, Y., Liu, J., Song, W., Chen, X., Luo, Q., & Liu, L. (2018). Production of β-alanine from fumaric acid using a dual-enzyme cascade. ChemCatChem, 10(21), 4984–4991.
Zhang, Y., Gu, H., Shi, H., Wang, F., & Li, X. (2017). Green synthesis of conjugated linoleic acids from plant oils using a novel synergistic catalytic system. Journal of Agricultural and Food Chemistry, 65(26), 5322–5329.
Lepak, A., Gutmann, A., Kulmer, S. T., & Nidetzky, B. (2015). Creating a water-soluble resveratrol-based antioxidant by site-selective enzymatic glucosylation. ChemBioChem, 16(13), 1870–1874.
Schmolzer, K., Lemmerer, M., Gutmann, A., & Nidetzky, B. (2017). Integrated process design for biocatalytic synthesis by a Leloir glycosyltransferase: UDP-glucose production with sucrose synthase. Biotechnology and Bioengineering, 114(4), 924–928.
Pei, J., Chen, A., Dong, P., Shi, X., Zhao, L., Cao, F., & Tang, F. (2019). Modulating heterologous pathways and optimizing fermentation conditions for biosynthesis of kaempferol and astragalin from naringenin in Escherichia coli. Journal of Industrial Microbiology & Biotechnology, 46(2), 171–186.
Baroja-Fernandez, E., Munoz, F. J., Li, J., Bahaji, A., Almagro, G., Montero, M., Etxeberria, E., Hidalgo, M., Sesma, M. T., & Pozueta-Romero, J. (2012). Sucrose synthase activity in the sus1/sus2/sus3/sus4 Arabidopsis mutant is sufficient to support normal cellulose and starch production. Proceedings of the National Academy of Sciences of the United States of America, 109(1), 321–326.
Schmolzer, K., Gutmann, A., Diricks, M., Desmet, T., & Nidetzky, B. (2016). Sucrose synthase: a unique glycosyltransferase for biocatalytic glycosylation process development. Biotechnology Advances, 34(2), 88–111.
Oka, T., Nemoto, T., & Jigami, Y. (2007). Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-D-glucose to UDP-L-rhamnose conversion. The Journal of Biological Chemistry, 282(8), 5389–5403.
Brazier-Hicks, M., Evans, K. M., Gershater, M. C., Puschmann, H., Steel, P. G., & Edwards, R. (2009). The C-glycosylation of flavonoids in cereals. The Journal of Biological Chemistry, 284(27), 17926–17934.
Falcone Ferreyra, M. L., Rodriguez, E., Casas, M. I., Labadie, G., Grotewold, E., & Casati, P. (2013). Identification of a bifunctional maize C- and O-glucosyltransferase. The Journal of Biological Chemistry, 288(44), 31678–31688.
Nagatomo, Y., Usui, S., Ito, T., Kato, A., Shimosaka, M., & Taguchi, G. (2014). Purification, molecular cloning and functional characterization of flavonoid C-glucosyltransferases from Fagopyrum esculentum M. (buckwheat) cotyledon. The Plant Journal: for Cell and Molecular Biology, 80(3), 437–448.
Hirade, Y., Kotoku, N., Terasaka, K., Saijo-Hamano, Y., Fukumoto, A., & Mizukami, H. (2015). Identification and functional analysis of 2-hydroxyflavanone C-glucosyltransferase in soybean (Glycine max). FEBS Letters, 589(15), 1778–1786.
Funding
This work was supported by the National Key R&D Program of China (2017YFD0600805), the National Natural Science Foundation of China (31570565), the Natural Science Foundation of the Jiangsu Province (BK20160929), the Open Foundation of Jiangsu Provincial Engineering Laboratory for Biomass Conversion and Process Integration (JPELBCPI2017002), and the Qing Lan Project and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pei, J., Sun, Q., Gu, N. et al. Production of isoorientin and isovitexin from luteolin and apigenin using coupled catalysis of glycosyltransferase and sucrose synthase. Appl Biochem Biotechnol 190, 601–615 (2020). https://doi.org/10.1007/s12010-019-03112-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03112-z