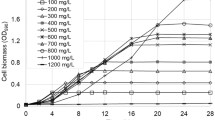
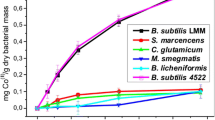
Abstract
A rigorous knowledge of the bacterial growth kinetics is essential for the scaling-up and optimization of biodegradation process conditions in a bioreactor. Although a great deal of literature is available on the modeling of bacterial growth kinetics considering the inhibition at high substrate-loading, the inhibition caused by toxic metabolic byproducts was not accounted in the bacterial growth kinetics. This work primarily aimed at developing a parametric bacterial growth model to account for metabolite inhibition, indicated by a decelerating log-phase growth, which was rarely discussed in the previous studies. An efficient azo-dye degrading bacterium (Bacillus subtilis MN372379) was isolated from the sludge-waste nearby a carpet-dyeing unit. The isolated bacterial strain was used to decolorize the simulated wastewater containing Congo red dye. This study proposed a computational approach to calculate specific bacterial growth rate time-averaged over the entire sigmoidal log phase (including the decelerating phase) for incorporating the effect of metabolite-inhibition, in contrast to the conventional studies where only the initial part (accelerating) of log phase was considered. The nature of metabolite inhibition was also determined and found to be non-competitive. Next, the computed time-averaged specific bacterial growth rate was incorporated into three substrate inhibition models to account for both, the metabolite and substrate inhibitions, and subsequently their kinetic parameters were also determined. Finally, the initial dye concentration and inoculum size were optimized to yield maximum dye utilization rate. This study paves the way for predicting bacterial growth kinetic with improved accuracy to enable a better optimization of bioreactors at the industrial scale.








Similar content being viewed by others
Availability of Data and Material
Not applicable
Code Availability
Not applicable
References
Kalyani, D. C., Patil, P. S., Jadhav, J. P., & Govindwar, S. P. (2008). Biodegradation of reactive textile dye Red BLI by an isolated bacterium Pseudomonas sp . SUK1. Bioresource Technology, 99(11), 4635–4641.
Bhatia, D., Sharma, N. R., Singh, J., & Kanwar, R. S. (2017). Biological methods for textile dye removal from wastewater : A Review. Critical Reviews in Environmental Science and Technology, 47(19), 1836–1876.
Banerjee, U. C., Rai, H. S., Bhattacharya, M. S., Singh, J., Bansal, T. K., & Vats, P. (2005). Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Critical Reviews in Environmental Science and Technology, 35, 219–238.
Koyani, R. D., Sanghvi, G. V., Sharma, R. K., & Rajput, K. S. (2013). Contribution of lignin degrading enzymes in decolourisation and degradation of reactive textile dyes. International Biodeterioration and Biodegradation, 77, 1–9.
Belkacem, M., Khodir, M., & Abdelkrim, S. (2008). Treatment characteristics of textile wastewater and removal of heavy metals using the electroflotation technique. Desalination, 228(1-3), 245–254.
Hartmann, M., Kullmann, S., & Keller, H. (2010). Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. Journal of Materials Chemistry, 20(41), 9002–9017.
Mahmoud, H. R., El-Molla, S. A., & Saif, M. (2013). Improvement of physicochemical properties of Fe2O3/MgO nanomaterials by hydrothermal treatment for dye removal from industrial wastewater. Powder Technology, 249, 225–233.
Somensi, C. A., Simionatto, E. L., Bertoli, S. L., Wisniewski, A., & Radetski, C. M. (2010). Use of ozone in a pilot-scale plant for textile wastewater pre-treatment: physico-chemical efficiency, degradation by-products identification and environmental toxicity of treated wastewater. Journal of Hazardus Materials, 175(1-3), 235–240.
Chaturvedi, A., Rai, B. N., Singh, R. S., & Jaiswal, R. P. (2021). A comprehensive review on the integration of advanced oxidation processes with biodegradation for the treatment of textile wastewater containing azo dyes. Reviews in Chemical Engineering (published online ahead of print 2021), 000010151520200010.
Ekambaram, S. P., Perumal, S. S. & Annamalai, U. (2016). Decolorization and biodegradation of remazol reactive dyes by Clostridium species. 3 Biotech, 6, 1–8.
Ghoreishi, S. M., & Haghighi, R. (2003). Chemical catalytic reaction and biological oxidation for treatment of non-biodegradable textile effluent. Chemical Engineering Journal, 95(1-3), 163–169.
Paz, A., Carballo, J., Pérez, M. J., & Domínguez, J. M. (2017). Biological treatment of model dyes and textile wastewaters. Chemosphere, 181, 168–177.
Yaseen, D. A., & Scholz, M. (2018). Treatment of synthetic textile wastewater containing dye mixtures with microcosms. Environmental Science and Pollution Research, 25(2), 1980–1997.
Tazdaït, D., Abdi, N., Grib, H., Lounici, H., Pauss, A., & Mameri, N. (2013). Comparison of different models of substrate inhibition in aerobic batch biodegradation of malathion. Turkish Journal of Engineering and Environmental Sciences, 37, 221–230.
Machado, K. M. G., Compart, L. C. A., Morais, R. O., Rosa, L. H., Santos, M. H., De Santos, U. C., Centro, F., De Minas, T., & Horizonte, B. (2006). Biodegradation of reactive textile dyes by basidiomycetous fungi from brazilian ecosystems. Brazilian Journal of Microbiology, 37(4), 481–487.
Sankaran, S., Khanal, S. K., Jasti, N., Jin, B., Pometto, A. L., & Van Leeuwen, J. H. (2010). Use of filmentous fungi for wastewater treatment and production of high value fungal byproducts: a review. Critical Reviews in Environmental Science anf Technology, 10, 400–449.
Niu, Q., Zhang, Y., Ma, H., He, S., & Li & Y. Y. (2016). Reactor kinetics evaluation and performance investigation of a long-term operated UASB-anammox mixed culture process. International Biodeterioration and Biodegradation, 108, 24–33.
Tan, Y., Wang, Z. X., & Marshall, K. C. (1996). Modeling substrate inhibition of microbial growth. Biotechnology and Bioengineering, 52, 602–608.
El-Sheekh, M. M., Gharieb, M. M., & Abou-El-Souod, G. W. (2009). Biodegradation of dyes by some green algae and cyanobacteria. International Biodeterioration and Biodegradation, 63(6), 699–704.
Baranyi, J., McClure, P. J., Sutherland, J. P., & Roberts, T. A. (1993). Modeling bacterial growth responses. Journal of Industrial Microbiology, 12(3-5), 190–194.
Monod, J. (1949). The growth of bacterial cultures. Annual Review of Microbiology, 3(1), 371–394.
Sponza, D. T., & Işik, M. (2004). Decolorization and inhibition kinetic of Direct Black 38 azo dye with granulated anaerobic sludge. Enzyme and Microbial Technology, 34(2), 147–158.
Talaiekhozani, A., Jafarzadeh, N., Fulazzaky, M. A., Talaie, M. R., & Beheshti, M. (2015). Kinetics of substrate utilization and bacterial growth of crude oil degraded by Pseudomonas aeruginosa. Journal of Environmental Health Science and Engineering, 13, 1–8.
Lineweaver, H., & Burk, D. (1934). The Determination of Enzyme Dissociation Constants. Journal of American Chemical Society, 56(3), 658–666.
Waldrop, G. L. (2009). A qualitative approach to enzyme inhibition. Biochemistry Molecular Biology Education, 37(1), 11–15.
Arutchelvan, V., Kanakasabai, V., Elangovan, R., Nagarajan, S., & Muralikrishnan, V. (2006). Kinetics of high strength phenol degradation using Bacillus brevis. Journal of Hazardous Materials, 129(1-3), 216–222.
Geed, S. R., Kureel, M. K., Giri, B. S., Singh, R. S., & Rai, B. N. (2017). Performance evaluation of Malathion biodegradation in batch and continuous packed bed bioreactor (PBBR). Bioresource Technology, 227, 56–65.
Kureel, M. K., Geed, S. R., Giri, B. S., Rai, B. N., & Singh, R. S. (2017). Biodegradation and kinetic study of benzene in bioreactor packed with PUF and alginate beads and immobilized with Bacillus sp. M3. Bioresource Technology, 242, 92–100.
Monteiro, Á. A. M. G., Boaventura, R. A. R., & Rodrigues, A. E. (2000). Phenol biodegradation by Pseudomonas putida DSM 548 in a batch reactor. Biochemical Engineering Journal, 6(1), 45–49.
Tsai, S. L., Lin, C. W., Wu, C. H., & Shen, C. M. (2013). Kinetics of xenobiotic biodegradation by the Pseudomonas sp. YATO411 strain in suspension and cell-immobilized beads. Journal of Taiwan Institute of Chemical Engineers, 44(2), 303–309.
Andrews, J. F. (1968). A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnology and Bioengineering, 10(6), 707–723.
Aiba, S., Shoda, M., & Nagatani, M. (1968). Kinetics of product inhibition in alcohol fermentation. Biotechnology and Bioengineering, 10(6), 845–864.
Edwards, V. H. (1970). The influence of high substrate concentrations on microbial kinetics. Biotechnology and Bioengineering, 12(5), 679–712.
Agarry, S. E., Audu, T. O. K., & Solomon, B. O. (2009). Substrate inhibition kinetics of phenol degradation by Pseudomonas fluorescence from steady state and wash-out data. International Journal of Environmental Science and Technology, 6(3), 443–450.
Sen, S., & Sarkar, P. (2019). Modelling of growth kinetics of isolated Pseudomonas sp. and optimisation of parameters for enhancement of xanthine oxidoreductase production by statistical design of experiments. Journal of Environmental Science and Health - Part A: Toxic/Hazardous Substances and Environmental Engineering, 54(1), 65–78.
Talha, A., Goswami, M., Giri, B. S., Sharma, A., Rai, B. N., & Singh, R. S. (2018). Bioremediation of Congo red dye in immobilized batch and continuous packed bed bioreactor by Brevibacillus parabrevis using coconut shell bio-char. Bioresource Technology, 252, 37–43.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549.
Victor, H., Ganda, V., Kiranadi, B., & Pinontoan, R. (2020). Metabolite Identification from Biodegradation of Congo Red by Pichia sp. KnE Life Sciences, 5(2).
Shah, P. D., Dave, S. R., & Rao, M. S. (2012). Enzymatic degradation of textile dye Reactive Orange 13 by newly isolated bacterial strain Alcaligenes faecalis PMS-1. International Biodeterioration and Biodegradation, 69, 41–50.
Author information
Authors and Affiliations
Contributions
Conceptualization: Anuj Chaturvedi and Ravi P Jaiswal. Methodology: Ravi P. Jaiswal and Ram S. Singh. Formal analysis and investigation: Anuj Chaturvedi. Writing—original draft preparation: Anuj Chaturvedi and Ravi P. Jaiswal. Writing—review and editing: Anuj Chaturvedi, Ram S. Singh, and Ravi P. Jaiswal. Funding acquisition: Ram S. Singh and Ravi P. Jaiswal. Resources: Birendra N. Rai and Ram S. Singh. Supervision: Ram S. Singh, Birendra N. Rai, and Ravi P. Jaiswal.
Corresponding author
Ethics declarations
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The participant has consented to the submission of the case report to the journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaturvedi, A., Rai, B.N., Singh, R.S. et al. A Computational Approach to Incorporate Metabolite Inhibition in the Growth Kinetics of Indigenous Bacterial Strain Bacillus subtilis MN372379 in the Treatment of Wastewater Containing Congo Red Dye. Appl Biochem Biotechnol 193, 2128–2144 (2021). https://doi.org/10.1007/s12010-021-03538-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03538-4